Special state of matter: superfluid helium
1988/04/01 Legarreta, J. A. Iturria: Elhuyar aldizkaria
all this has long coincided with the daily experience, in the triology appeared some error, as the known as pasta state. It has no stiffness of solids or fluidity of the lees, as it has intermediate characteristics. Despite doubts about the triology of aggregation states, no one cared about this issue.
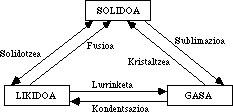
Through improved techniques, researchers had the opportunity to work at high temperatures and found an ionic state that resulted in the second gaseous state called plasma. Then astronomers, along with the discovery of neutron stars, resulted in a second solid state called hyperdense matter. The ability to work at very low temperatures resulted in the second liquid state, called superfluid state. He challenges the most consistent laws of the liquid state, whose interpretation, even at the atomic level, is very difficult and discussed.
In this little work we will talk about the superfluid state of helium, the rarest superfluid physicists have found.
Characteristics of helium
It was discovered by astronomers in the sun and was later identified among the rarest gases in the atmosphere. The helium that can be found in the atmosphere is mainly 4 2 He, being the isotope 3 2 He in a percentage of 10-7%.
In 1868 helium was discovered in the atmosphere of the sun thanks to the spectrograph. It could be achieved by heating certain minerals in the earth. Until the exploitation of natural gas deposits, helium production was very small, but sufficient for laboratory needs.
At the atomic level, although that of helium after hydrogen is very simple (two protons, two neutrons, two electrons), as we have said before, it is the superfluid that adopts the rarest behavior.
Superfluous helium
By 1900 researchers began to liquefy all known gases.
As is known, any gas can be liquefied and solidified under suitable conditions by cooling up to a certain temperature. Moreover, the higher the pressure on the gas, the higher the liquefaction temperature. For example, propane and butane can be liquefied by simple compression in a bottle at room temperature. Therefore, in the liquefaction of a gas we can take advantage of both pressure and temperature to achieve our goal.
Despite working at very low pressure in practice, liquefaction of most gases requires very low temperatures, with an absolute zero limit (-273.16°C = OK). In fact, atmospheric pressure oxygen requires a temperature of -218°C, nitrogen -210°C, chlorine -101°C and neon -248°C. Despite the difficulty of liquefaction of other gases, over time and especially due to advances in the technique, the liquefaction of all gases was obtained. One of the gases that presented this difficulty was hydrogen, whose liquefaction temperature (-260°C) is very low. Therefore, the liquefaction temperature of hydrogen, very close to absolute zero, greatly hindered the researchers, obtaining James Dewar in 1892.
As we all know, when molecules are stagnant (i.e. when there is no molecular movement), kinetic energy and therefore temperature take the zero value. In absolute zero there is no molecular movement. Therefore, no molecule can reach an absolute zero state.
So, as we said at the beginning of this little work, we will stand in the helium. In 1900 all gases except helium were liquefied. Although all the liquefaction pathways of helium were worked, on the one hand, it could not be liquefied and much less solidified, and on the other, the researchers who did it thought that it could only be in a gaseous state, regardless of its pressure and temperature value.
In fact, the first statement is correct, since only by cooling the helium cannot be solidified. And as for the second statement, it must be said that it is not a sustainable gas, but the most difficult to liquidate. The liquefaction of helium was obtained by the Dutch physicist Kammerling Onnes in 1908. Therefore, all gases could be liquefied by paying what it meant to work at low temperature. This discovery encouraged researchers to learn the characteristics of liquid helium. Since they began to investigate these characteristics they saw that they were very surprising, unable to explain their behavior.
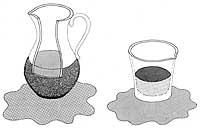
1) The superfluid enters through the wall of the porous container until the inside and outside superfluid are at the same height.
2) If we generate an instantaneous electric current through an electrical resistance, the heating would affect the internal superfluid, penetrating inside the container to cancel the change of the external superfluid.
3) Due to the flow of superfluids from the outside of the container to the inside of it, a stream of superfluids is generated.
For starters, the liquefaction temperature of 4 2 He is the smallest known at normal pressure (1 atm): -268,82°C (4,18K), i.e. four degrees Celsius above absolute zero. 3 Compared to He 2, the liquefaction temperature is lower: -270°C (3,2K). Although the temperature decreases completely, up to 0,001K, for example, under environmental pressure the two isotopes of helium cannot be solidified.
The liquids obtained at the indicated temperatures have the characteristics of all liquids, but if the temperature decreases, both reach the state of superfluous. 3 The He reaches the superfluid state at temperature 0,001K. As for 4H, it reaches its superfluid state at a temperature of 2.17 K. Today, Finns have the right tools to get such low temperatures and keep them for a long time. In the following lines we will focus especially on the characteristics of the 4 atomic weights helium isotope, which presents the most peculiar characteristics.
This helium isotope produces a liquid at a temperature of 4,18K, called He 1. As mentioned above, a decrease in temperature around the temperature of 2,17K causes the He undergoes a curious transformation to which a change in its physical characteristics occurs. This transformation seems solidification, but it remains liquid. If the temperature of 2.17 K is reached, the 4He takes a superfluid state and this new form is called He II.
What are the peculiarities of Helium II?
Above the wall
Viscosity is the property of all fluids, both gases and liquids. Viscosity represents the internal friction of the fluid, which transmits the effects of a movement through the fluid by joining the direction of the fluid. Unity is the poem (P).
The viscosity of water at 25°C is 0.01 P. Pasty liquids have a very high viscosity and crystals are infinite. On the contrary, gases have very low viscosity. Superfluid helium has no viscosity, i.e. it is completely fluid (perfect fluid).
For example, a drop of oil slides through a glass more slowly than a drop of water. Therefore, oil has a higher viscosity than water. But the known superfluid helium drops much faster than any normal liquid. A drop of superfluid helium would slide through the crystal as fast as a ball of lead falling into the air from a certain height.
On the contrary, unlike normal liquids, any object moving within superfluid helium does not find any friction.
According to a fluid mechanics law, the larger the moving diameter of a fluid, the faster it flows. On the contrary, He II adopts a totally opposite behavior; in small diameter tubes it moves faster than in large ones. This behavior leads us to a curious experiment: if we filled an unglazed ceramic jar with superfluous helium, it would go very fast through the wall. The water would remain inside the jar. Therefore, He II is a fluid so perfect that it passes through the microscopic pores of the burnt earth.
But if we kept it in a porous container, what would happen? It could be thought that He II would remain there, that is, he would not leave because there are no pores. However, this does not happen; it begins to climb up the inner wall of the container and goes down the outer wall as if each molecule were to leave climbing a wall. The process continues until emptying the container.
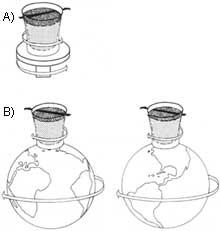
B) It remains still with respect to the stars that serve to fix the astronomical coordinates. The ship rotates with the Earth when the superfluid helium is completely stopped.
This process would not be carried out by any normal liquid, since on the one hand the viscosity prevents the capillary displacement of the sheets of the liquid and by the surface tension the liquid tends to fill the smallest possible volume and on the other hand it should overcome the force of gravity without receiving energy from the outside. Here we introduce the second principle of thermodynamics, which is that the self-borrowing system always loses energy, starting with the situation of greatest disorder.
Thus, He II overcomes all the aforementioned drawbacks (viscosity, surface tension, gravity and second principle of thermodynamics). The process would approach that a layer of small thickness of the superfluid begins to rise through the inner wall covering it completely. Arriving at the edge of the boat begins to descend through the outer wall. At this time the outer layer, under the influence of gravity, pulls the inner layer, emptying the container. Therefore, in the process He II overcomes the barriers of the normal liquids mentioned above.
Does not like twist
If we place water or any other normal liquid in a container and turn the container, both vertically and horizontally, the water will also begin to rotate on the turning axis and with centrifugal force a swirl will be formed around the axis. This does not happen with helium II: If we turned the full container of He II in a vertical direction or in any other direction, the superfluid would remain still.
Moreover, not only with the laboratory, but also with the stars standing, until the laboratory itself turns with the Earth in Space. The superfluid, therefore, stands completely in relation to the fixed directions taken as a reference in astronomy. Although the movement of the ship in any direction (of course, slow turns and transfers are taken into account and the ship is not agitated or overturned), the He fulfills this characteristic. In this experiment He II is a stable reference to all the movements of the container and behaves like a gyroscopic spinning top.
Since superfluid helium has no viscosity, it has no adhesion or force resistant to displacement with the walls of the vessel, that is, by not affecting superfluid helium, it fulfills the principle of inertia. First, let's look at what this principle says: a material point that is not influenced by any force stands or moves with a uniform rectilinear motion. As a consequence of this prince we have the property of matter called inertia. This property indicates that a body cannot naturally change its state of motion or rest. Since superfluid helium is not transmitted by friction, He II remains motionless according to the principle of inertia, once the container has begun to move.
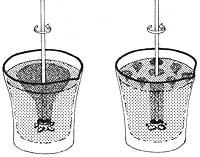
On the other hand, if superfluid helium were to move, according to the principle of inertia, it should move with a uniform rectilinear motion, since, as indicated above, there is no force resistant to displacement. This statement can be demonstrated by the following experiment: since the He II collapses inside a long, closed tube, the He II will move continuously in the tube making a permanent internal current, just as the permanent electric currents move in superconductors.
Heat behaviour
When heat spreads easily inside a body, it is said to be a good heat conductor. On the contrary, it is a bad heat conductor when it is difficult to expand. Therefore, when a body conducts heat easily, it has a high thermal conductivity. Thermal conductivity is the property that tells us how a body carries heat.
In fluids, each molecule can move from one point to another, that is, it moves. This movement spreads heat through the fluid.
When a normal liquid is subjected to a heat source, the closer any point of the heat source liquid is, the hotter it is, that is, the liquid is heated locally. Therefore, differences are generated within the liquid. Heat extends from hot spots to colder ones, and the greater the difference between points, the faster and faster.
Helium superfluid does not behave as it has been said before in the heat. On the one hand, its thermal conductivity is infinite, and this is increasing, the smaller the difference between points. On the other hand, as there is no hot spot on the bottom of the superfluid helium, it does not boil. However, when heated it aromatizes, but evaporation occurs on the surface, leaving the rest of the fluid at the same temperature. In practice, therefore, it appears as a heat superconductor, that is, if small differences occur, the heat spreads quickly through the liquid. Therefore, local heating or cooling is practically impossible. Therefore, inequalities cannot occur within the liquid.
To know the propagation of heat through superfluous helium, the following experiment was devised: if a container with pores and tight neck is inserted in a superfluous helium bath, superfluous helium penetrates through the pores, ending the penetration process when they are at the same external and internal height. If we subsequently heated the inside of the container with an electrical resistance, the superfluid that surrounds it would enter through the wall to cool the inside and maintain balance. Due to the amount of helium that enters the container, the large stream of helium leaves the neck of the container to eliminate the difference in mass.
Researchers have rebuilt this experiment by creating short heats based on an electrical resistance. These heating causes heat to expand like waves through the liquid mass. These waves are called secondary echoes. This form of heat propagation is not met in any other condition.
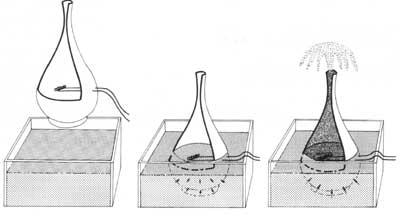
case of using a glass or metal container, the superfluid helium goes up the inner surface of the wall and, once it reaches the edge of the container, descends through the outer surface of the wall, gradually emptying the container and dispersing the superfluid on the table.
Conclusion
Among other things, all experiments performed with 4 He could be performed with 3 He, but it would be necessary to work at cooler temperatures, 0,001K around the temperature. 3 He has very surprising properties: it can be solidified under certain pressures, but not cooling, but heating. In addition, superfluid 3 He is magnetic, like iron or nickel, and the experiments performed demonstrate two types: 3A and 3B. 3A Helium density (with greater magnetisation) is very difficult to calculate due to its variability. Density varies depending on the magnetic field that crosses the superfluid.
Finally, the superfluous situation deeply interests astronomers, as it explains the behavior of neutron stars. In them there is a layer of neutrons that is not solid, liquid or gaseous, but superfluous. Therefore, the studies being carried out in cryoscopic laboratories lead us to a sky that is not affected by matter.

Gai honi buruzko eduki gehiago
Elhuyarrek garatutako teknologia



