Car pollution
1989/09/01 Otaolaurretxi, Jon | Tapia, Xipitri Iturria: Elhuyar aldizkaria

Cars generate more pollution than any human activity. This is not new, since since the end of the second world war it has been a problem that has been growing year after year. The first regulatory regulation known for this problem was developed in the US in 1963 and in 1983 most countries in the world accepted the standard polluted air figures. The Netherlands, Japan, Hegokore, Austria, Sweden, Novergia and Switzerland have taken new steps in anti-pollution regulations. In April of this year the European Parliament called on the European authorities to apply the standards approved in the US in 1983, as European car manufacturers have not treated too much in this field.
Some international organizations claim that gasoline and diesel engines emit at least half of carbon oxides (II), hydrocarbons and nitrogen oxides from fossil fuels. In addition, these engines emit other types of particles.
Carbon oxide (II) can cause death in closed premises. Nitrogen oxides facilitate the formation of certain harmful acids. Some hydrocarbons can also cause cancer.
If the engines emit the aforementioned substances, combustion is insufficient. The combustion that occurs in the bulls pistons requires four factors: high pressure, high temperature, fuel and oxygen. The piston compresses the evaporated fuel mixture and air. In gasoline engines, when the piston reaches the top of the cylinder, a spark extracted from a spark plug causes the explosion of the mixture. As the pistons are joined by a connecting rod, the crankshaft begins to spin.

This movement is transmitted to the wheels of the cars and therefore moves. If the performance of the engine was 100%, the fuel of the cylinders would be burned in its entirety, but not so, in gasoline engines the performance ranges between 65% and 75%, and in the cranks between 80% and 90%.
The main causes that combustions are not complete are on the one hand that in the cylinder not all points are at the same temperature. On the other hand, air nitrogen can form oxides in cylinders and fuels, which contain sulfur and nitrogen oxides, cause strong explosions inside the cylinders and form oxide layers in the valves.
Therefore, if you want a better combustion, you need to deal with the problems mentioned. And that is not impossible. Petrochemical companies can remove impurities from fuels and engineers must technically guide the use of lead-free fuels.
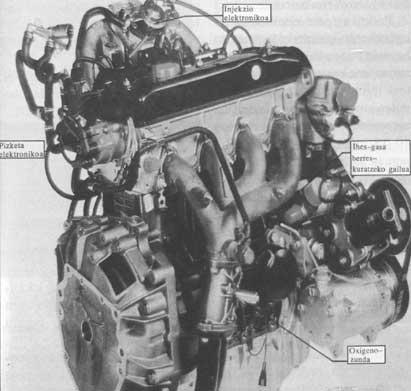
As for the engines, the first thing to do is to improve the carburetor. The function of this device is to mix fuel and air before entering the cylinder. Better than carburettor is the injector. The injector, in the fresh air chamber, introduces evaporation under pressure. Electronic devices measure the amount of fuel the engine needs. The next step is for the explosion to occur at the necessary time.
The explosion time varies according to what the engine needs and electronic devices are needed to control the moment of the explosion in each cycle. However, there is an obstacle, which is economic, which is expensive. The car does not offer, much less, the most suitable conditions to work.
Another way to improve combustion can be the recycling of exhaust gases. Obviously, the quality and finish of the different materials that make up the engine have to do with the performance of the engine.
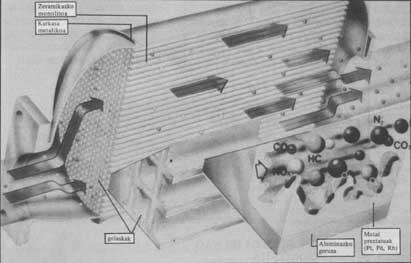
The function of the catalytic canister is to transform carbon dioxide (CO) into carbon dioxide (CO), nitrogen oxide into nitrogen and burn totally unburned hydrocarbons. Inside this stainless steel boat, the burned gases in the engine pass through some platinum group metals (palladium or rhodium), which can accelerate (catalyze) chemical reactions without their participation.

In practice, the catalytic can only has a stainless steel housing and inside a ceramic block called monolith. The monolith has thousands of small cells of about a millimeter of side and on the ceramic support has an aluminum layer of 10-15 microns thick. This layer combines palladium, platinum and rhodium microcrystals. The thousands of inerts are due to the fact that the burnt gases touch the catalysts at their exit on the largest possible surface (about 2.8 m 2).
In the three precious metals mentioned above, rhodium is the most interesting by itself, as it affects the three pollutants. However, it costs disfiguratively (about 6,000,000 pesetas per kilo), so only a small piece of rhodium is usually placed. Most contain platinum. Whether hydrocarbons are burned or CO gas becomes CO 2, the kilo is worth “only” 2,600,000 pesetas. Palladium has the same effect, but is of lower yield (it costs approximately 600,000 pesetas). Normally in the catalytic boat there is usually 15% rhodium, 55% platinum and 30% palladium mixture.
In new gasoline cars with a displacement of more than two liters in Europe since October last year, the catalytic boat is mandatory, which increases the price of the car by about 100,000 pesetas.
In smaller cars, catalytic boats are expected to be mounted gradually, with the intention of obligatory them to place them in all new cars in 1993.
In small displacement cars, the catalytic boat has no rhodium. The CO gas will be converted into CO 2 and the unburned gases will be completely burned. Catalytic oxidation needs more oxygen and therefore fresh air is injected into the pot through a pump. To reduce the amount of nitrogen oxide, the engine needs a more accurate carburettor. The excess air entering the cylinder increases the combustion temperature and increases the generation of nitrogen oxides. To improve the installation some of the exhaust gases can be reincorporated into the cylinder due to the decrease in the cooking temperature and the lower generation of nitrogen oxides.
In large displacement cars (more than 2 liters) the catalytic boats will have the three metals mentioned above. To properly catalyze the reaction of the gases, its composition must be kept constant. This means that the air/gasoline mixture that enters the cylinder must be very dosed and that the mixture must be lit at a very specific time. The first condition is obtained by electronic injection of gasoline. This injection is governed by a calculator with a probe called lambda that reports the oxygen from the gas going to the boat. 14.5 grams of air and one gram of gasoline is the ideal stoichiometric ratio or theoretical mix. If you have more air, not all nitrogen oxides can be oxidized in the catalytic canister and if you have more gasoline, unburned hydrocarbons will not be completely eliminated in the canister.

It is advisable to govern electronically the moment to turn on the mixture inside the cylinder, but large displacement cars have electronic ignition and do not have to solve this problem.
The precious metals contained in the catalytic canister do not intervene directly in chemical reactions. Therefore the life of the pot has no limits theoretically. In practice, however, from 80,000 or 100,000 kilometers the performance of the boats decreases considerably. The precious metal microcrystals are also agglomerated and the contact surface in the boat is smaller.
No Super gasoline! This is another requirement required by the catalytic boat. The boat does not support leaded gasoline. Therefore, since “super” gasoline has lead (the normal has less) it should not be used. By burning “Super” gasoline lead oxides are extracted in the exhaust gases and leaves the monolith’s inerts full. Some additives added to crankcase oil also contain metal particles and are prohibited in case of jars.
Therefore, the obligation to place catalytic boats must be accompanied by the consumption of unleaded gasoline Eurosuper. This new gasoline requires a lower compression ratio in the cylinder and the engine power is about 3% lower. Consumption increases at the same time.
The catalytic boat has another danger. If by engine breakdown the fuel and air mixture passes through the cylinder without burning, it burns in the electrolytic pot and its temperature can reach up to 1400C. At this temperature the inner monolith melts and deteriorates.
In order to avoid all these disadvantages that the catalytic boat has, the solution would be to buy diesel engine cars. As for carbon oxide (II) and nitrogen oxides, the diesel engine complies with all European standards, but other harmful contaminants are generated, such as sulfur dioxide and unburned particles, which seem carcinogenic. No good filters have yet been invented against these harmful pollutants.
Another alternative would be the “poor mix” or “layered” engine. This engine has a lower compression ratio in the cylinder, better performance, more complete combustion and less contaminants in exhaust gases. But it has been twenty years since this type of engine was prepared and nobody has yet sold it.
Therefore, research can focus on many lines. It is possible to restore and clean the environment, but today it is expensive. Technology and the economy are closely linked. Health damage is also costly (casualties, missed days, hospital care, etc. ). ). In short, buying polluting cars or expensive cars depends on our economic ability.

Gai honi buruzko eduki gehiago
Elhuyarrek garatutako teknologia




