Hydrogen bonds New horizons in chemistry
1997/11/01 Irazabalbeitia, Inaki - kimikaria eta zientzia-dibulgatzaileaElhuyar Fundazioa Iturria: Elhuyar aldizkaria
Abdeza studied chemistry at the University of Marrakech and has since researched in prestigious research centers around the world. Among these centers is the prestigious CALTECH of California. He is currently a professor at the University of Castilla-La Mancha.

During the talk, Professor Abdezarra Duhal highlighted the peculiarities of the hydrogen bond, its importance and the results of the latest research on it. Linus Pauling defined it as the basis of the strong hydrogen bond some sixty years ago. In fact, this type of bond, in which a hydrogen atom makes a bridge between the two heteroatoms, is key in many biological processes. For example, in the DNA helix the two auxiliary chains are joined by hydrogen bonds of nitrogen bases.
One of the main features of hydrogen bonds is its flexibility and the latest research on hydrogen bonds has yielded tangible results. Professor Duhal summarized the research results on three main ideas.
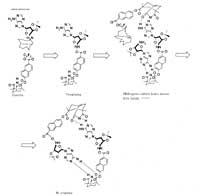
On the one hand, since hydrogen bridges bind DNA chains, it is important how hydrogen bridges are formed, that is, the hydrogen atom and its union. In fact, when the bases are joined by pairs only some hydrogen bridges are allowed, so adenine is only associated with thyminia and cytosine to guanine. However, due to the flexibility of the hydrogen bond, all possible spatial configurations of the link are in continuous dance. At the time of DNA repetition, if the bond configuration, spatial disposition, is erroneous, DNA repetition is erroneous because the proper bases are not associated (guanine is associated with adenine rather than cytosine, for example) and, consequently, incorrect genetic information is transmitted to the new DNA molecule, which can lead to the formation of a cancer.
On the other hand, it is very difficult to obtain information about the instantaneous configuration of the hydrogen bond by directly analyzing the DNA molecule. Therefore, obtaining adequate molecular probes to obtain this information would be a great advance in the simple analysis of the molecular mechanisms of biological processes. The basis of these probes can be, for example, the special characteristics of the flexibility of hydrogen bridges, the absorption of light at certain frequencies and the possibility of measuring this light with spectrometry.
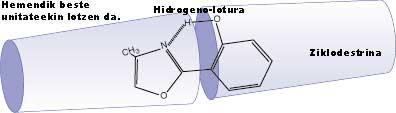
Secondly, some molecules with internal hydrogen bonds due to the flexibility of the hydrogen bond can be used in biological processes in specific environments, such as at the points of influence of drugs, in the form of a probe, to study the configuration of these sites and in the case of drugs, for example, to obtain information to increase their effectiveness. This feature also allows them to locate molecules within container-type molecular structures, such as cyclodextrins. These vessels, in turn, can be arranged in the form of interconnected tubes and these molecules that contain the hydrogen bond existing inside can adopt special configurations, being able to absorb and emit light at certain frequencies, which can serve to transmit information through this tube through the light.
Finally, the techniques used in the study of hydrogen bonds, femtosecond lasers, can make chemical reactions visible throughout the entire period of reaction development, that is, what is happening at all steps of chemical reaction. Until fifty years ago, only reagents and products were seen in chemical reactions. Then there was the possibility of seeing some intermediaries, but these techniques also show transition states and all the dynamics of the chemical reaction can be exposed.

Gai honi buruzko eduki gehiago
Elhuyarrek garatutako teknologia