Advances for lithium-ion batteries
2010/10/01 Elhuyar Zientzia Iturria: Elhuyar aldizkaria
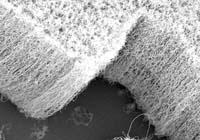
In recent times, two studies have been published showing a significant increase in energy storage capacity by using unusual components in lithium-ion batteries.
In one of them, the cobalt oxide electrode typical of batteries has been replaced by a carbon nanofrio at the Massachusetts Institute of Technology (MIT). In this way, they have managed to supply five percent of the energy that conventional lithium batteries can provide, which has allowed this power to be supplied up to ten times faster.
In lithium-ion batteries, positively charged lithium-ion batteries accumulate in the graphite electrode during battery charge and move from graphite electrode to lithium-cobalt oxide electrode. They form chemical bonds with the oxygen atoms of the lithium-cobalt oxide electrode, resulting in an electric current.
The electrode generated at MIT would not work if carbon nanotubes were intact, as lithium ions would not join them. However, researchers have added different types of oxygen compounds to the surface of nanotubes, forming layers of nanofrio. They are very porous layers, that is, they occupy a large surface the compounds with oxygen, which allows the union of many lithium ions. That's why they can provide power five to ten times faster than conventional lithium batteries.
In the other study, researchers at the Pacific Northwest National Laboratory have found that the addition of paraffin and oleic acid facilitates the formation of plate-type lithium-manganese phosphate nanostructures. The use of these nanoplates as components of the electrodes allows the introduction and extraction of electrons and ions in a very simple way. Thus, the material that itself does not serve as a battery material has become a magnificent energy tank.
In fact, they have found that it can store 10% more energy than lithium phosphate electrodes, commonly used in hybrid and electric cars.

Gai honi buruzko eduki gehiago
Elhuyarrek garatutako teknologia




