Lithium batteries: high expectations and difficulties
1991/12/01 Azkune Mendia, Iñaki - Elhuyar Fundazioa Iturria: Elhuyar aldizkaria
All batteries manufactured by Alessandro Volta in the next 50 years after the invention of his battery were for one use. When they were discharged they were thrown because they could not be reloaded.
However, the French physicist Gaston Planté invented in 1859 a battery that could be recharged again and again by introducing lead plates in sulfuric acid, a system that is still basically used in cars and trucks.
In these classic batteries there are two electrodes: one the anode (positive pole) and the other the cathode (negative pole), both immersed in a solution called electrolyte. The anode is a metal to which the electrons (negative charges) come out. These electrons are used in the outer circuit (for example, illuminating the car's lamp). The remaining positive charges (metal ions) along the electrolyte reach the cathode inside the battery.

Between these two poles of battery charged wiring outside, lamps, resistors, electric motors, etc. The use of the accumulated energy is obtained by its start-up. However, when that accumulated energy is spent, the battery must be recharged and for this purpose it must be circulated to the anode in reverse. In the opposite case, instead of extracting energy, you have to give the tension backwards.
With the aim of getting fast and massive energy transport systems in a small space, electrochemicals are opening up new ways to capture large amounts of ions and electrons. They want to multiply the contact surface of the current classic cathodes by taking advantage of the “intercalate structure”. The intercalate structure is that of some solids that are usually classified in thin leaves. In nature, mimics are the best known example. They have stable and thin homogeneous sheets according to a plane, but when the plane is joined it is weak; it is like the cake of millileaves.
The idea is to create a new structure by intercaling thin sheets with others of material. If in addition to this mechanical operation electrons are released in the electrochemical reaction, the cathode will have a large contact surface and will accumulate a lot of energy. In addition the process must be reversible (it must be reloaded once downloaded) and cycles must be repeated many times.
The development of such systems began mainly in the Bell laboratories in the United States in 1974-75, and since then there are numerous groups of researchers who are working in this field. As in many other topics, military applications have given a great boost to the development of intercalate structure batteries, and in the batteries that currently contain lithium sheets, it can be said that in the same weight it accumulates between three and five times more energy than in the classic ones.
In these batteries it is very interesting that the anode is lithium, since the lithium atoms are very small. The Li+ ions that have lost electrons in the chemical reaction with the electrolyte can circulate between very narrow cathode sheets at short intervals. The miniaturization of the battery is very simple.
In addition to lithium anodes, the researcher has rolled the laminar structures of the cathode. Although the structure of the stacked sheets is very comfortable to imagine, the researchers have studied other structures. These structures can have their organization in one dimension (fibers), in two dimensions (plates) or in three dimensions (crystalline structure).
The fibers present the barrier of the tunnels, which consists in that an interior obstacle breaks all traffic. There are no ribs to fix the problem. It must be taken into account that, above all, it is intended that within the cathode a significant displacement of many lithium ions occurs, which in short means that in the outer utilization circuit we will have more electrons or more current. In this sense, two-dimensional or three-dimensional structures are more interesting, although in turn they are more complex.
Numerous sessions have been held in the United States and Europe with titanium disulfide (IV) (TiS 2). If you look at titanium and sulfur atoms using electronic microscopes, you see basic three-dimensional structures that are repeated; in the case of TiS 2, octahedra. However, these octahedrons are joined regularly by forming sheets. Each sheet of titanium atom is among the other two of sulfur atom, as if it were in sandwich. The weak space between the plates, technically called “Van der Wals hollow”, is between two layers of sulfur atoms.
In this structure, despite some specific errors in practice, it surprises the mobility of Li + ions, which is similar to that of liquids. On the other hand, it is surprising that, theoretically, when the Li + ion has conquered its leafy, the anode has eaten completely.
Although the theory seems attractive and encouraging, the difficulties to exploit industrially these lithium batteries are not few. For a few years, lithium batteries have been manufactured industrially. In wrist watches, the mercury batteries compete, and what you're wearing now may also be. Lithium anode and manganese oxide cathode (MnO2). But as is evident, these batteries are not recharged and the process is not reversible.

To be reversible there are difficulties in the cathode aspect. And it is that rarely the charge is insufficient: the battery must be charged and discharged hundreds of times. In the anode there are also difficulties in reversibility. After several cycles the dentrites appear between the sheets and cause harmful short circuits.
In 1979 the American manufacturer Exxon launched a new battery making a cathode with titanium disulfide (IV), but the results were unfortunate, since it only allowed to charge five times.
In 1984 the Canadian firm Moli Energy extracted a molybdenum disulfide battery (IV). It is true that it could be charged 500 times, but this type of batteries is too sensitive to temperature and is not energetically interesting compared to nickel/cadmium batteries.
The manufacture of sulfide compounds for cathode is expensive and dangerous. Obtaining 100 grams in the laboratory is simple, but with a kilo of weight the risk of explosion is high. Therefore, what measures should be taken for industrial use!
Another of the difficulties is the electromotive force or tension that must be reached between the poles. With lithium anodes and sulphur cathodes it is achieved between 1.3 and 2.5 volts (2.1 V with the cathode of TiS 2), which is insufficient when they want to form batteries of 12 or 24 volts as in cars.
At present it seems that the metal oxide cathodes are more successful. For example, manganese oxide cathode (IV) (MnO 2) is cheap and an electromotive force of about 3 V is obtained. Sony and Sanyo work on this path. In France, for its part, the cathode has been made with vanadium oxide (V 2 O 5) (anode with lithium, of course), obtaining an electromotive force of 3.2 volts. The process is reversible if the battery is not discharged too much. Unlike lead batteries, these lithium batteries do not support the discharge up to 0 volts. It is recommended that the aforementioned vanadium batteries are being loaded and unloaded between 2.8 and 3.8 V.
Although the electromotive force is adequate with metal oxide cathodes, the current intensity must compete with classic batteries. The energy stored and supplied by kilogram (W.h/kg or W.h/dm 3) is the most interesting fact. Nickel/cadmium are of another system, but they circulate around 100 W.h/dm 3 and it is expected that with nickel/hydride this figure will rise up to 150. In lithium/vanadium, they reach 175 W.h/dm 3. The truth is that it is not bad energetically.
However, on the one hand it can only be charged 100 times and on the other the electrolyte used has risks. The electrolyte is a solid lithium salt (LiAsF 6 ). The formula shows that the electrolyte involves arsenic and we all know that it is very toxic.
Although so far we have spoken about the anode and cathode, the electrolyte or anodo-electrolyte phase is the third field in which difficulties must be overcome. Electrochemicals also have the word.
However, for the moment it seems that the electric car will not be powered by lithium batteries. In addition to the energy required for this, lithium is an expensive material (8,000 pesetas per kilo) and in the car is charged and discharged thousands of times the battery.
In the future, lithium batteries are expected to have applications in space and in the military sphere, where the price is not contemplated so much. Otherwise it can be said that they will be used in phones, t-shirts (camcorder with integrated magnetoscope), computers, etc. Japanese industries are working at least.
CLASSIC CAR BATTERY
It has two electrodes submerged in sulfuric acid or electrolyte. The positive anode or electrode is lead and also cathode or negative, but has a lead oxide layer (IV).
When electric current is sent to the outer circuit, the following process is performed. Anode lead reacts by ionizing with sulfuric acid. The lead atom releases two electrons (2e - ) and becomes Pb ++. Sulfuric acid is also disassociated with two positive H+ ions and a negative OS 4 ion. The reaction result is the formation of OS 4 — and OS 4 Pb by combining Pb ++. The liberated electrons run the outer circuit and after performing their work (lamp ignition, for example) they reach the other electrode.
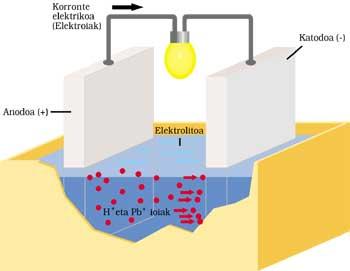
The two electrons from the lead oxide cathode (IV), sulfuric acid, two H+ ions from the previous reaction and anode, supply lead sulfate and two water molecules.
After operating time, part of the electrolyte becomes salt and water and the lead oxide layer (IV) is spent. The current supplied by the battery is increasingly weak, so it must be charged. For this it is necessary not to extract energy to the battery and deliver it. OS 4 Pb is broken down by application in the first electrode of Pb and OS 4 H 2. In the other electrode OS 4 Pb is broken down and Pb 2 and OS 4 H 2 is removed.
LITHIUM BATTERY
It has two electrodes immersed in electrolytes. The elements of the anode and cathode have been placed parallel to each other by layers and all have been collected. The anode is a pure lithium, the electrolyte is solid lithium salt (LiAsF 6) and the cathode parallel sheets of vanadium oxide (V 2 O 5).
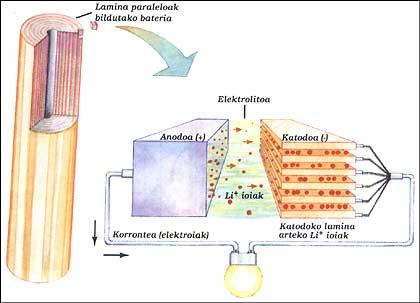
When the battery supplies the outer circuit, the electrons of the anode run the circuit, while the positive ions leave the anode inside, cross the electrolyte and head to the lamines of the cathode structure. When you have to charge the battery, the ions and electrons work backwards, for this you have to give it energy as in the classic battery.
This system allows to obtain, at the same power, sizes between 3 and 5 times less than nickel/cadmium batteries. This advantage is because lithium is a very small atom. This allows lithium ions to circulate between very close sheets.

Gai honi buruzko eduki gehiago
Elhuyarrek garatutako teknologia