Myth of Therapeutic Cloning: Truths and Lies
2002/02/01 Escajedo San Epifanio, Leire - Konstituzio Zuzenbideko IrakasleaEHU Iturria: Elhuyar aldizkaria
When all this was about to relax, Antino told the American Academy of Sciences that before it ended this year (2001), the cloned man would be created. As is known, this project received severe criticism, but in the end the day of Antino has arrived: Advanced Cell Technology (ACT) has affirmed that it has managed to clone a human embryo. But, where are they now so much and so much against cloning? According to the ACT, it is a "therapeutic" cloning that should not be confused with "human repetitive cloning." This explanation has calmed down for moments the criticisms.
But, if it is so good, why is it banned in most European countries? We believe that there is little truth and many confusions among which have been said about "therapeutic" cloning. The confusion has also arisen, intentionally, because behind it there are, besides the scientific interest, important economic interests. We would like to make a little light about it, provide some data so that society has the right tools in its ethical reflection.
What is 'therapeutic' cloning?
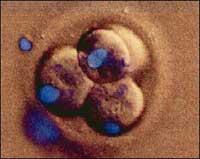
The cloning procedure has been added the name of therapeutics to differentiate it from the cloning that is done for regeneration purposes. As for the cloning procedure, however, the 'therapeutic' cloning used by the ACT was performed through the nuclear insertion, that is, the same technique used for the creation of the Dolly sheep. It cannot be said, therefore, that we are before two different types of cloning. The procedure is unique and consists of replacing the nucleus of an embryo with the nucleus of an adult cell. What the ACT has achieved so far can be compared with the first phase of Dolly's creation process. The difference, therefore, would be in the second phase. That is, in the posterior use of the cloned embryo.
If the cloned embryo were viable, it could be implanted in a woman's belly and develop a cloned child. The cloned embryo can, therefore, be used for ‘regeneration’. Another option is to remove cells from this cloned embryo and develop with them a therapy for the cloned person. More than a therapeutic cloning, we should talk about cloning for therapy.
Cloning for regeneration XIX. There are scientists who since the twentieth century have wanted to equip themselves to many other techniques that have been created to deal with the sterility of couples, but it must be said that they are a minority within the scientific society. Techniques of artificial fertilization older XIX. They are from the twentieth century. These techniques knew important advances in the twentieth century, such as the birth of Louise Brown and the 'child who has come from the cold', born of a cryopreserved embryo. The peculiarity of cloning is that all the characteristics of the child can be chosen, since by cloning it is intended to give all the genetic characteristics of another person who has already existed. Since the birth of Dolly this technique is believed to someday be applicable to humans.

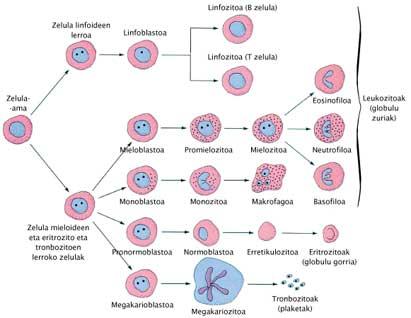
However, therapeutic cloning aims to develop the cloned embryo, but not from it until the birth of a child. When the blastocyst is formed from the preembryo, some cells are collected inside: embronic stem cells or embryo stem cells. It is believed that with these cells, therapies can be developed to cure or replace diseased cells in some human beings. Once the cells are taken, the cloned embryo does not serve us at all and if you want it can be removed.
Before rehearsing with stem cells from embryos, scientists have made great discoveries with stem cells from adult beings, especially bone marrow and umbilical cord blood. Stem cells are cells that have mammals. From them you can get any separate cell. For example, cells obtained from the placenta allow rebuilding the blood and immune system of the child with leukemia. Hematins from people with sickle cell anemia and treating children born with a strong immunodeficiency may also be restored.
The peculiarity of stem cells from embryos, compared to others, is that they are totipotent and can be regenerated indefinitely. For example, the number of cells obtained from the placenta is small to be used as therapy for an adult, but with the cells collected from the embryos, this shortage could be overcome due to the in vitro proliferation of the latter. This is the case of animal trials.
The realization of these sessions with human embryos is fundamental to continue investigating in this line, but it must be said that the embryo for obtaining stem cells does not have to be a clone. Stem cell research is broader than therapeutic cloning, and should be expressed in public debates.
The therapeutic cloning is aimed at the phase of application of a therapy developed with stem cells. Since the therapy to be applied is, in short, a kind of cell vaccine, it is believed that the therapy will be more successful if the origin of the medicinal cells is the embryo of the clone of a person. In this way, the risk of rejection would be overcome. However, it has not yet been sufficiently demonstrated with animals. The amount of rejection is unknown and if the quality of the cells of the cloned embryo can be adequate to overcome it. On the other hand, it must be taken into account that the cloning process can lead to unknown risks.
In summary, we want to highlight some criteria. First, the inadequate separation between therapeutic cloning and regenerative cloning. It aims to promote the distinction between 'good' and 'bad' cloning, in order to achieve the legalization of one of them. But we believe that to allow this legalization, society must make that decision freely, with the right information. Before judging the usefulness of therapeutic cloning, we must discuss whether the embryo is for us a human being, an empty cell, or something else.
Finally, it should be noted that the cloned embryo of the ACT has remained in the first phases of cell division, without generating the morula, so the creation of a child would not be viable. It does not serve to develop therapies for the moment, since we have to wait for the stem cells to arrive at the blastocyst situation to receive them. Why have they made it public? As these investigations can only be promoted in the United States with private subsidies, perhaps a little bit of advertising comes wonderfully.
Possibilities for the advancement of therapeutic cloning in our society
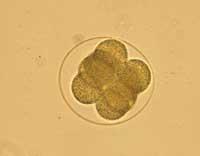
With the exception of the United Kingdom, in all the countries of the European Union, a profound legislative change would be necessary to allow research and testing with embryo cells, more so the creation of cloned embryos.
When Dolly was born in 1997, the founders of the Council of Europe's Biomedicine Convention drafted a special Protocol prohibiting human cloning. The Protocol was signed by 19 members of the Council of Europe on 12 January 1998, including France, Portugal and Italy. Very few countries have signed but ratified this Convention, only in force in Greece, San Marino, Slovakia, Slovenia and Spain. The prohibition of cloning is, moreover, very narrow. It does not expressly prohibit cloning, but through any procedure a man is created genetically identical to a person, alive or dead.
The techniques of cloning not related to regeneration, although with the same techniques, are not regulated by this law. Thus, somehow, it can be seen that “cloning for regeneration” and therapeutic cloning also want to be separated into laws. Germany and France have done the same in their legislations. They prohibit and punish the creation of identical human beings by cloning, but do not speak of 'therapeutic' cloning.
If so, why is therapeutic cloning not investigated in Europe, except in the UK? Easy. Because although therapeutic cloning is not expressly prohibited in the laws regulating the "embryonic status", there are some of the actions that require its procedure. At this time, only the United Kingdom accepts research with embryonic cells, including therapeutic cloning. It highlights, however, the case of Germany. There it is not possible to 'create' embryonic cells, but their purchase abroad and use in research is legal.
In all other countries there are great obstacles. Sometimes research with viable embryos is prohibited, as in Spain. Although it has been investigated elsewhere with cryopreserved embryos, it is forbidden to create new embryos, except for regeneration, or choose the sex of the embryo. Therapeutic cloning would be indirectly prohibited in these cases by the need to create an embryo in an express way and by the sex of the repeater.
In any case, most of the laws that exist globally in this regard, regardless of whether or not research is approved, have in common a number of important criteria. The embryos used in scientific sessions cannot be applied in the womb of a woman so that a child can be born from her. This does not prevent therapeutic cloning, but does the prohibition criterion for developing the embryo in vitro after 14 days from fertilization. Research in the development of embryonic cells is fundamental to know the capacities of these cells in therapeutic cloning. According to this rule, these cells can be developed, but once past day 14, cells are stored, but the embryo should be removed.
What can happen from now on?
The therapeutic cloning and the own research with embryonic cells have at this time great obstacles. It is clear that in one way or another these investigations will advance, but the welcome of society cannot be foreseen.
Research with embryonic cells continues to be scarce and, for the most part, have been carried out in the last five years. In addition, it must be taken into account that these investigations have been carried out with the participation of few countries, since in most countries they are prohibited. Despite this, these investigations go very quickly and it is difficult to know their fruit correctly.
In this situation we cannot ask society to issue a quick (and positive) judgement on therapeutic cloning, because the doubts about this problem remain many. The process of social acceptance of any discovery should be calm and deep, as occurred with the recognition of in vitro fertilisation. Only thus can the benefits and risks of a technique be detected and measures taken to avoid improper uses.
For our part, we do not believe that therapeutic cloning should be legalized at this time. The use of cloning is nothing more than a type of cloning, so studying nuclear integration in human embryos would be possible, although laws prohibit, creating clonic human beings. This risk, in our opinion, is excessive at the moment, since its usefulness will not give us much service until the embryonic cell therapies are developed correctly. These therapies, as has already been indicated, can be investigated with non-cloned embryos.
The previous step would, therefore, be to legalize the research carried out with embryonic cells. The British Department of Health presented in its report many of the possible profits of these investigations and scientists have begun to investigate. The research is initiated and, therefore, we are in time to adapt its regulation.
Probably this phase begins with the granting of research permits with cryopreserved embryos in most countries. This has already been requested by the National Commission for Assisted Reproduction, despite the opposition of the Vatican. Its prior use for the benefit of science is considered more appropriate than the elimination of unused embryos in fertilisation processes. These research would be limited to two limitations, both mentioned above: that after its use in research, embryos cannot be used for reproduction and after 14 days of fertilization, the embryo cannot be developed in vitro (although the cells do). It would be appropriate to promote these studies with public grants, so that all the results of the investigations are reflected.

During this phase, it must be taken into account that the therapies developed in cloned animal models are striving. In a few years, therefore, we will learn more about the benefits and risks that cloning brings to stem cell therapy. By then, we may know what effects genetic errors can have in embryo cells, or if mitochondrial DNA, part of DNA that is not modified in cloning, can cause problems.
Consider that if we speak of legalizing the 'therapeutic' cloning, we must first demonstrate that cloning has therapeutic capacity. Otherwise, once the cloning is authorized, we cannot avoid its use in regeneration. The prohibitions and sanctions imposed in a few countries have little force compared to other interests that move our world. Therefore, before accepting the "therapeutic" cloning of an embryo, it will be necessary to see if the "therapeutic capacity" of this technique that abuses the processes of nature can go from a myth to a reality.
Process of discussion of therapeutic cloning
At the end of last year, the UK declared its intention to legalize stem cell research, including therapeutic cloning. When he learned of this intention, the Parliament of the European Union asked him for withdrawal, and that same petition was made by the Pontifical Academy of Sciences and ethics committees of many countries. Six months later, this legalization came into force, arguing that this technique was the door to new therapies. To develop therapies that complement diabetes II, for example, bituminous cells that produce insulin in rats have been obtained in the laboratory.
Shortly afterwards, George Bush commented on two things about his research with embryonic cells, which were released live on television stations around the world. Looking ahead, he noted that the law prohibiting any kind of cloning had begun to be discussed at the United States Congress. Therefore, Congress has given its approval to the law, but the U.S. Senate has paralyzed the bill to know the opinion of scientists. As for the stem cells of the uncloned embryos, Bush authorized them to follow the investigations that were in progress at the time, but to those who were not initiated, for the moment he has not given them permission to start.
International responsibilities have been different in recent months, and summer events had somehow been forgotten until the publication that a human embryo has been "cloned". Apparently, with the aim of performing a therapeutic cloning, all the benefits that are added to embryonic cells have been associated with this new one and the Ministry of Science and Technology itself has shown its willingness to 'approve' this type of cloning. This is surprising because the Spanish state prohibits and punishes each and every research with embryos, plus the most cloned. Since 1988 about 50,000 embryos have been frozen and, according to law, they cannot be eliminated or used for research. Therapeutic cloning already exists before legalizing it.

Gai honi buruzko eduki gehiago
Elhuyarrek garatutako teknologia