Text written in Basque and translated automatically by Elia without any subsequent editing. SEE ORIGINAL
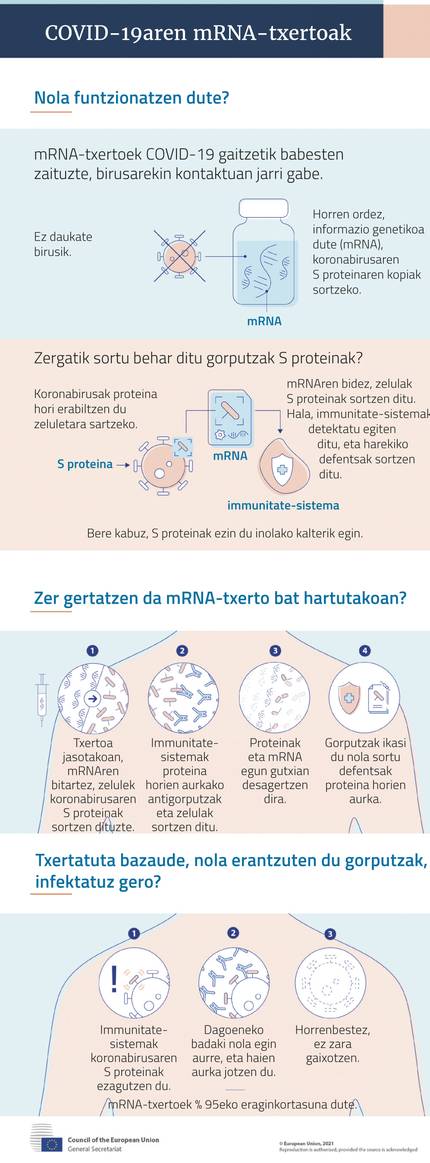
RNA messenger, essence new vaccines
The first two vaccines authorized by the European Medicines Agency to protect against COVID-19 are based on the messenger RNA. Thanks to their characteristics, they have demonstrated their efficacy and safety before classic vaccines. However, its development has not ceased and other vaccines based on messenger RNA are being carried out, among which are Isabel Sola Gurpegi and her co-workers, in the Spanish Centre for Biotechnology.
Vaccines are key in the fight strategy against COVID-19. Thanks to them, vaccinated people have trained their immune system to prevent the proliferation of the virus and SARS-CoV 2 infection.
Classic vaccines achieve this by internalizing pathogen, both weakened and inactive, as triple viral vaccine and polio. Others, like those of Hepatitis A and B, carry antigenic proteins of the pathogen, that is, they generate immune response.
In addition, there are also genetic engineering for the transformation of harmless viruses such as adenobiruses, which incorporate genetic information for the production of antigenic proteins. Ebola and dirty are of this type.
All these strategies are being developed in different laboratories around the world to respond to covid-19. However, the first to be installed in Europe are not of the type mentioned above. Moreover, outside the laboratories, they are completely new. And now two have been authorized, compared to other types. They are vaccines based on messenger RNA (MRNA vaccines).
Annual evolution
Scientists have been researching DNA vaccines for years and some are already testing it in people like HIV or rabies. In the case of Koronabirus, studies conducted in 2000 with SARS-CoV1 and later with MERS-CoV suggested that this technology could be useful.
All have the same base: they have as raw material the mRNA molecule that encodes the production guidelines of antigenic proteins. When this MRNA penetrates the cells, it produces antigenic protein in the ribosomes of the cells, which when it appears on the surface of the cells, reacts the immune system and produces antibodies and cell response. With this, the vaccinated person is protected.
In their experience with SARS and MERS, they claimed that the key is the S protein (spike, spicle) found in the crown wrap. Through it, the cells that infect the virus are accessed. Therefore, covid-19 MRNA vaccines carry guidelines for the production of protein S.
However, they have had to overcome some difficulties. On the one hand, mRNA can cause a disproportionate inflammatory reaction, so it has had to change the sequence so that it does not occur. One of the main changes is the substitution of nucleoside uridine by pseudouridine: genetic information does not vary, but there is no risk of inflammation.
On the other hand, its easy decomposition has led to other modifications to stabilize it. And to make your immune response even stronger. Finally, methods have been developed to be introduced into the cells of the body and prevent them from rapidly degrading, collecting and protecting.
Specifically, they have created lipid nanocaps. In addition to protecting mRNA molecules, they help them enter the cells by fusing them with the surface of the cells. To some extent they reinforce the immune response. In exceptional cases they can cause a severe allergic reaction (anaphylaxis), so people with this risk cannot get a MRNA vaccine.
Safe and efficient
Once these obstacles are overcome, they occur easily and quickly, since they do not need to grow in eggs or cells like other vaccines. Thus, at 66 days after the publication of the genome of SARS-CoV2 by the Chinese researchers, the first American volunteers received the first puncture of the experimental vaccine developed by Moderna. In May the first results were announced and then the sessions of Pfizer-Biontech were cited.
The results of Phase III of the clinical sessions were even better than expected: Both have shown an effectiveness close to 95% and Moderna is even better than Pfizer-Biontech to protect against serious symptoms.
The rest have similar characteristics. For example, both need two doses: The modern ones of 100 micrograms, with a range of 28 days, and those of Pfizer-Biontek of 30 micrograms, of 21 days.
Both must be kept cold, but the lipid wrappers of both are not equal. Thus, Pfizer-Biontechena needs -70ºC and only lasts 5 days in the refrigerator. On the other hand, the Moderna is sufficient to keep it at -20ºC and has a duration of 30 days in the refrigerator. This facilitates logistics, especially in those areas where resources are not excessive.
Millions of doses of these MRNA vaccines have already been implanted in several countries, including Euskal Herria, and more will arrive. One of them can be the one developed by the German pharmacy company Curevac. Biodonostia and Biocruces participate in the III edition of the clinical sessions. In phase, and like the previous ones, it is based on the mRNA protein S. However, they have advanced that it has certain advantages with respect to others: they do not need such a low temperature to conserve, it occurs in Europe and is one of the most doses it has acquired in Euskal Herria and in the countries of its environment.
Self-replication and sterilizer
In addition to MRNA based S-protein vaccines, more complex MRNA vaccines such as the Spanish Centre for Biotechnology (CNB-CSIC) are being developed. Isabel Sola Gurpegi is co-director of the native coronavirus laboratory, and according to her, the main characteristic of the vaccine being developed is its self-replication.
The fact that the vaccine is autoreplicative has an obvious advantage: it generates several copies of the incoming molecule. This makes the doses of the vaccine much lower and, therefore, the production is also more economical.
In addition, the information carried by the mRNA is much more complete. The previous vaccines only contain information for the production of the protein S, while those that are being investigated in the Sola laboratory also carry other proteins. “We have seen that these proteins also generate a significant immune response. Thus, the immunity generated by our vaccine is more complete, powerful and balanced than that obtained with others, and may also be more sustainable,” explains Sola.
On the other hand, other vaccines have shown that the infection of the vaccinated person prevents the disease. However, they do not know if they also prevent the replication of the virus, so despite being vaccinated there may be a risk of infection. To achieve a sterilizing immunity, that is, capable of preventing the replication of the virus, the response that occurs should be very powerful.
The application mode also influences the sterilizing capacity. Sola has specified that the current inserts are intramuscular, and that is what are prioritizing regulatory entities, which is the most classic. “We are going to try two ways: the intramuscular and the nasal. Why? Because the intrainnose facilitates the obtaining of sterilizing immunity”.
In fact, SARS-CoV2 penetrates the body through the nasal and pharyngeal membranes, and from there it descends into the respiratory system towards the bronchi and lungs. “If the vaccine is administered through the nose, for example by means of a spray, the immunity is exerted on the respiratory membrane, that is, at the entrance door of the virus. If we breathed the virus, that local immunity would respond immediately through IgA antibodies, which would close the way to the virus. Immunity would therefore be sterilizing,” he explained.
In animals it has already been shown that the immunity produced by the nose is sterilizing: the protection is 100% and the virus disappears. In addition, being self-replicative, it has been affirmed that it is sufficient with a single dose.
Only necessary genes
To make it self-replicable they use the characteristic of viruses. Soles: Soles: “Viruses are intrinsically self-replicative and therefore contain specific proteins: the self-replication machinery. We have a long history researching the coronavirus and among the methods we have developed we have a system of reverse genetics to transform the virus genome. With this, we have removed from the SARS-CoV-2 genome all harmful genes (genes that help you move from one cell to another, cause inflammation, etc.) And we have only left those who need to self-replicate and promote the response of the immune system.”
Therefore, the MRNA vaccine is replicated by the natural virus machine and makes thousands and thousands of copies of antigens. This allows a more complete and powerful protection than the previous ones. “The previous ones carry about 4,000 nucleotides of the protein S, while ours has about 20,000”.
Two forms of DNA vaccine they have created are being tested. One, like the previous ones, with nanoparticles and another with the same mRNA. To do this, they replicate their mRNA in some cells. With this the mRNA obtains the protein necessary to produce the packaging. Result: viral particles (VLP).
These VLP have the same external appearance as SARS-CoV-2 and assimilate them into cells. When they are inside, however, they cannot spread to other cells, as they do not have the genes to produce the proteins they need. This system is called “suicidal”: when entering the cell it replicates, but cannot be extended to other cells.
Sola acknowledges that, despite the good results obtained in the laboratory with this system, they may have difficulties for approval by regulatory bodies, for their innovative nature.
Although vaccinations have begun, there are still some unanswered questions, such as the duration of the immunity they produce and the degree of sterilization. Ed. Biontech.
Mutations, deadlines and incorporations
Asked if the virus mutations will affect the effectiveness of the vaccine, Sola has explained that MRNA vaccines produce the complete S protein and that the immune response they generate is contrary to many domains of the protein. Therefore, it is very difficult for mutations to revert the answer: even if you do not know any domain, you will react against others. However, if critical changes occur, the messenger mRNA should be changed using the same technology.
Now the tests will begin with humanized mice, then with primates and, at a good pace, they will begin clinical sessions in humans with the support of the CSIC and the Spanish Ministry of Health. If all results are favorable it does not exclude authorization for inclusion in 2021.
Thus, one more vaccine could be given. Solo has no doubts: “The more vaccines the better. Some may be more suitable than others for specific population groups. In this way, the inserts could be partially customized. On the other hand, we have the challenge to introduce ourselves all over the world: if we have different types of vaccines, which occur in different places, some cheaper and others more expensive and with different logistical characteristics, more easily they will reach everyone and all people.”
In any case, it is prudent and recalls that, even with authorized vaccines, there are still questions unanswered, such as the duration of the immunity they generate and the degree of sterilization: “Therefore, although it is integrated, it is essential to maintain preventive measures to avoid transmission.”
Buletina
Bidali zure helbide elektronikoa eta jaso asteroko buletina zure sarrera-ontzian