Uranyl ions react
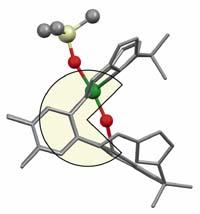
Uranium, the heaviest known element in nature and the long-standing radioactive element in nuclear waste, have reacted with an organic molecule at the University of Edinburgh.
They have reacted with the uranyl ion [UO2]2+. Because uranyl is very soluble in water, and the uranium-oxygen binding is extremely hard, the reactivity of the molecule is very low, making it very difficult to extract from water. If they managed to react with another compound and make it insoluble, it would be easier for this radioactive molecule to leave the environment.
The organic molecule involved in the reaction is shaped like a mouth, when joined with the uranyl slightly tilts the molecule properly straight. This small change triggers a chain of reactions.
Scientists know that with this reaction they will not be able to clean the water contaminated by uranium, among other things because it does not occur in the water. But they have said it will be useful to understand the behavior of the element.
Buletina
Bidali zure helbide elektronikoa eta jaso asteroko buletina zure sarrera-ontzian











