Research cuts, stop hope

It is not a new problem. In history, a part of society has often condemned scientific advances. One of the most controversial scientific developments of the last decade is regenerative medicine. Although in recent years more and more countries have recognized the great capacity of stem cell research, the controversy remains alive in other countries, such as the US. During President George Bush's term, they stopped collaborating with federal funds in stem cell research. President Obama reversed this situation in 2009 through an execution order, but in August of the same year a federal judge again blocked it and the government appealed it. Since then, controversy and legal exchanges have evolved and remain attentive to both scientists and the families of many sick people, who could benefit in the future from the results of research that has been interrupted by the veto.
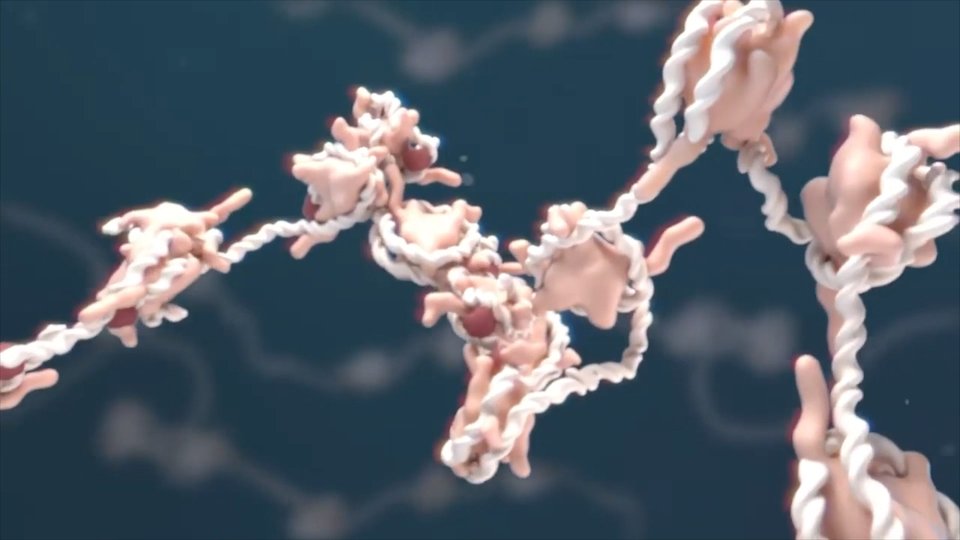
The aim of therapies based on regenerative medicine, supported by advances in stem cell technology and biology, is to form incurable wounds through traditional medicine and fully regenerate the structure of tissue as well as its functions. Currently, few therapies use stem cells and are widely supported by the scientific community. Adult stem cells are used in these therapies. For example, bone marrow transplants treat some blood diseases or restore the blood system after a therapy against certain types of cancer. The continuous research of embryonic stem cells has taken steps and some achievements have stimulated the optimism of many scientists and the hope of patients suffering from one of the diseases that today are incurable, such as the conversion of embryonic stem cells into cardiomyocytes (heart cells) or remarkable advances in diabetes.
The great economic investment made by the government as a future bet has contributed to the capacity and scientific progress of the last decades of the US, a support that has been different depending on whether the government is Democratic or Republican. In addition to federal funding, US laboratories have received informed and forceful financial support from individual legacies, private foundations and organizations of all kinds. This type of funding is very common in Anglo-Saxon countries. In some cases it is because donors have prioritized a type of disease (such as cancer or cardiovascular disease), but before that a team of independent experts should always confirm scientific excellence. Through this great and consistent economic aid, US laboratories have had a source of funding independent of the political and economic vicissitudes of the country, which has fostered their scientific and medical progress.
However, in many European countries the situation is different. In Spain, the support received by scientific research is limited almost exclusively to the support of the central government and the regional governments (corresponding body), a situation that also occurs in the Basque Country. As a result, changes in government and economic crises cyclically affect the essential funding of research, such as the current one, which has led to cuts. The recent reduction in the funding of laboratories in our country has already had practical consequences, since they have reduced the acceptance of projects, which has meant a reduction in work with available funds. Fewer experiments reduce scientific results, making medical advances in our country slower.
In the US, the resolution of the appeal to federal funding for stem cell research is probably a matter of time. Meanwhile, progress is constant thanks to the protection of private funds. In California (October 11, 2010), there has recently been an excellent example of the U.S. has opted for research, and specifically for embryonic stem cell research, with the aim of conducting the first global clinical trial. Last July, the FDA granted Geron the mandatory authorization for the clinical trial and now the first patient has been selected. Eight or nine other patients from seven American sanatoriums will join her. This patient has suffered major damage to the spinal cord and will receive a single injection of precursor cells from a type of nerve cells derived from embryonic stem cells. This trial will analyze in this first phase the safety and sustainability of the treatment. Subsequently, numerous tests are still needed to prove their effectiveness and admit paraplegic as a mobility recovery therapy. However, this company has been authorized for not receiving federal funding, so its work is not affected by legal discussions about the use of embryonic stem cells in research. Once again, private research support has enabled biomedical progress and stimulated the expectations of patients and their families.
We all want to see how that hope increases and how it improves the quality of life, but how do we get it without supporting research?
Buletina
Bidali zure helbide elektronikoa eta jaso asteroko buletina zure sarrera-ontzian