Virus strategies for cell invasion
1996/01/01 Nieva, Jose Luis Iturria: Elhuyar aldizkaria
Viruses are usually placed in the limit between living and inerts. The viriones do not own metabolic machinery, that is, they are not able to build by themselves their structural components from metabolic background. Therefore, in order to reproduce it is necessary to invade other beings (guests) and appropriate the metabolic machinery of the cells of them.
Within the genetic information carried by the viruses protected inside, the orders are accumulated to inhibit the metabolism of the infected cell and to guide the synthesis of the virus components. Therefore, viruses are molecular parasites that can sometimes become predators and lead to death mercilessly by being infected.
Therefore, to replicate, viruses are essential that your genome (DNA or RNA) enter the inside of the host. The process to follow for this is called an introduction. Since the entry is obligatory to be able to produce lnección, this has great importance in the life cycle of viruses. In the case of viruses that penetrate eukaryotic cells, virions must overcome the outer molecular cover or membrane surrounding the cell.
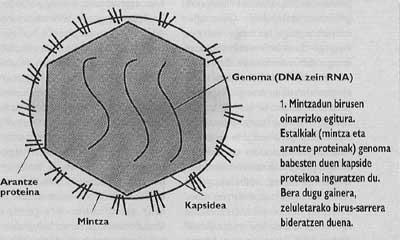
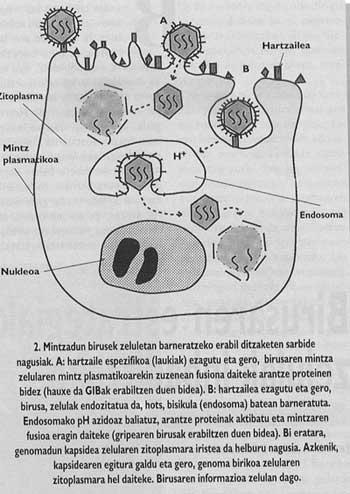
Likewise, the most important animals and viruses with the capacity to infect man are surrounded by a membrane like that of cells called covered (Figure 1). In order to guide the entry, these viruses have developed integration mechanisms with cell decks that will invade their own decks. Once the fusion of the target cell membranes and virion has been achieved, the virus genome can reach the cell cytoplasm (Figure 2) from where it begins to replicate.
The input strategies developed by the covert viruses are very complex processes that, to understand them well, scientists are making constant efforts, since if we could learn how to avoid the entry of the murdered enemy we would be in protection for certain infections. With the aim of finding a simple and global way to inhibit viral infections, researchers have sought the common elements of entry.
Unfortunately, things are not as simple as we want. As we know so far, at least every family of viruses has been developing a different input strategy, so the hypothesis that there is a general and common input mechanism has been losing strength. In addition, research on the mechanisms of entry of viruses has not yet yielded many results, since in the early 1980s, when scientists realized that it was not possible to produce a universal vaccine against the virus that produces the flu, this field began to develop and can be considered of recent creation.
In the words of the Spanish researcher Rafael Blasco: "Research into any virus is dead at the time the vaccine is developed to treat the disease it causes." Mr. Blasco is the most important specialist in the state in the introduction of viruses such as Poxvirus that creates smallpox. The virus of Navarre is the first being that human beings have intentionally disappeared from the planet, so Blasco says that there is not too much responsibility for the entry of their relatives who consider themselves to be unimportant.
The error of this approach has been revealed in recent times, without knowing how to stop the pests of flu and AIDS. Today it is not possible to predict the type of virus that will cause the next epidemic. To overcome this disability, it would be an important step to develop a universal therapy to treat any viral infection. To do this, it is also necessary to investigate in depth the virus entry processes. The flu virus, for example, is a specialist in trufar with our immune protection. In order to also neutralize viruses and other foreign agents, the defenses of the human body must know and differentiate previously these foreign agents. This is currently achieved through vaccines. The vaccines reveal the identity of foreign agents to the body's defenses, so that the cells that are going to produce antibodies that must be joined to these agents are ready before the infection occurs.
In the case of the flu, however, things are not so easy. Influenza vaccines have to be modified annually, since each outbreak of the flu virus has different antigens (i.e., different groups of viral molecules that can identify antibodies). What's more, it seems that the virus itself will change in the following generations knowing that it intentionally manifests these molecules. In this way we can produce antibodies against the virus with ease, but only the virus itself will know those molecules "maliciously exposed".
In other words, the protection offered by vaccines obtained by the use of ancient viruses or a particular slag has no value against new viruses or other slag. And of course, the nature or "identity" of the virus can be changed almost annually. On the other hand, it is evident that viruses do nothing of their own. Due to their high rate of replication, the selective processes usually influence with great intensity in the viruses and their evolution is usually slave. In this evolutionary process the types of virus favored are the most variable, since they usually have a greater facility to overcome the immune systems.
Thus, the researchers of the World Flu Center, located every year in Atlanta, are trying to find enthusiastically the identity of the person responsible for the next flu epidemic. Two or three months before the arrival of winter, the virus must be identified, purified and prepared in large quantities. Otherwise, an important part of the population made up of elderly, chronic patients, and other immunosuppressed people would be in danger (indirectly, flu causes more deaths annually than AIDS). In the case of flu, the importance of finding a different therapeutic alternative to the vaccine is evident.
But the best known example of the lack of success of vaccines is the vaccine against the viral agent of AIDS, which today enjoys unfortunate fame. This virus, called HIV (Human Immunodeficiency Virus), is a retrovirus type that in its life cycle gives a long space integrated in the cells of the guests. Therefore, after entering the host, the genetic information of the virus can be hidden between the genetic information of the cell and kept latent for several years (it is estimated that this situation can last for several years).
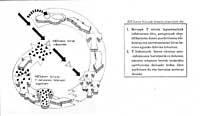
The main defense against this type of virus is the detection and destruction of infected cells, that is, the process called cellular immunity. On many occasions, invaded cells present in their skin fragments of foreign agent that, as it were, indicate their state. In these cases, specialized cells with flushing function detect these "symbols" and destroy infected cells. The cleansing cells are cytotoxic T cells. In infectious situations these cells need of other intermediate cells that allow the growth of them.
These cells, which fulfill the function of specialized intermediaries, are cedent lymphocytes, more specifically T4 lymphocytes. In addition, these lymphocytes are essential to coordinate other immune homicides. Unfortunately, T4 lymphocytes are favorite prey of the HIV virus and this defensive system not perfect in itself can become more defective if the HIV infection causes the disappearance of T4 lymphocytes that act as intermediaries. Thus, when the system collapses, an immunodeficiency occurs that causes the organism to be unprotected from any infection and can reach death.
This type of infection hinders the development of the vaccine. On the one hand, the vaccine would be a little effective cure for infected people, since antibodies can only detect virus assembled outside of cell work (i.e., with cover), since the HIV virus in latency is undetectable for our defenses. On the other hand, the most effective vaccines so far have been those prepared with weaker viruses, that is, vaccines that can cause small-scale infections. Obviously, a healthy person cannot be given this type of HIV vaccine, since the risk of irreversible and deadly infection is excessive.
To overcome this barrier, synthetic and recombinant vaccines are currently being studied. Although both models of vaccines are in the trial phase, in order to be effective in the future they must overcome a great obstacle: as in the case of the flu, the most important immunogenic characteristics of HIV are at the same time the most changing, of which they appear intentionally to show the virus itself. If we could differentiate the unalterable immune parts, we would have more possibilities to prepare an effective vaccine. For this, and as we will see later, it is necessary to investigate in depth the biology of the virus. Meanwhile, in the near future, the HIV vaccine seems like a dream. This was confirmed by experts who approached the World AIDS Congress held in Osaka in early August 1994.
The problem of influenza and AIDS pandemics leads us to investigate the biology of the viruses that cause these diseases. Both are covered viruses, that is, surrounded by a lipid membrane such as cell membranes (Figure 1). In fact, the virions steal the membrane of the cell that they are born. However, virus membranes do not contain the same proteins as the cell membrane, but special viral glycoproteins called spiny proteins. In these specific proteins could be the neck of the fight against viruses such as flu and HIV.
In the words of Judith White, the world's leading specialist in the introduction of the flu virus: "The proteins of thorns are essential machines for the entry, since these polyvalent proteins are those that deceive the immune system, they know the cellular receptor that limits the cellular tropism (what type of cell is going to invade) and they are superior to perform the functions of merging the cell and viral membrane." As will be seen later, these proteins are essential in this process, since blocking any of these functions could endanger the cellular penetration capacity of the virions.
Trying to stop entry can be a good method in the fight against viral infection. For this we need a good knowledge of the function and structure of thorny proteins. Once the functional parts of these proteins are known and the necessary factors for inclusion in the host are clarified, we can begin to design pathways that allow the interruption of the input. The thorny proteins of the HIV cover and flu viruses are paradigmatic in this regard. Its structure and functions are the most investigated (Figures 3-5).
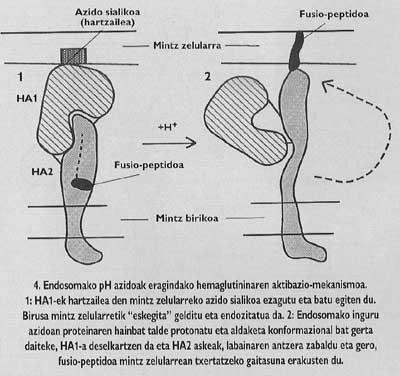
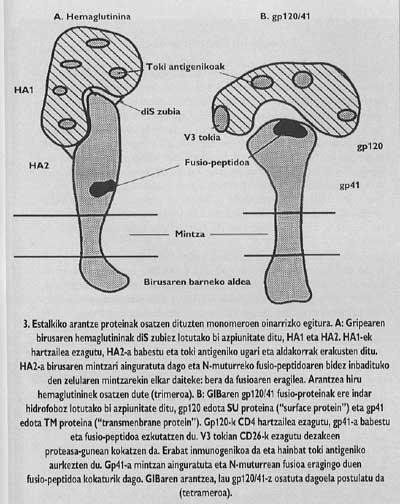
These membrane proteins have an oligomic structure, that is, they are formed by different modules. The spine protein of the influenza virus, hemagglutinin, is heterotrimer, that is, it is constructed with three monomers formed by two different subunits, HA1 and HA2. The subunit of the chapel is ha1 and the subunit with fusion peptide is ha2. The latter is the one that, when activated with the acidic pH of the endosoma, makes the fusion peptide appear and is integrated into the membrane of the target cell.
The hawthorn protein of HIV is tetric, that is, it is formed by four monomers called gp120/41. In this virus, the activation of the subunit with fusion peptide (called gp/41) is totally different from the flu virus. In order for the activation to occur, we must release this subunit of the subunit called gp120 that is in the hat, following a mechanism that until last year was not entirely clear, the Gp41 is released in two steps. The first is known and the CD4 receptor of the gp120 lymphocyte is integrated. Knowledge of CD4 is essential to initiate the process of fusion between membranes, but does not activate the fusogenic subunit. The lkerlaris found that GlBa does not infect rat cells with a human-like CD4 receptor generated by genetic engineering, although these mutant cells demonstrated their ability to attach to the virus equivalent to the T4 lymphocyte.
That is, although this CD4 receptor of mutant rat cells functioned as in humans (it was able to bind to the virus), its adherence to the receptor was not enough to channel fusion. Therefore, in addition to the CD4 receptor, another mysterious factor should influence the membranes of human T4 lymphocytes to make these cells hiv-sensitive. Therefore, it was postulated that HIV should have a second specific receptor. In autumn 1993, the Ara Hovanessia team at the Pasteur Institute found that it had identified this recipient.
It was formerly known that monofuntional antibodies created against the antigenic site V3 (Figure 3) on the surface of the Gp120 could stop the infection. The possible success of GP120 based synthetic vaccines currently being treated would depend on the protection that could offer antibodies capable of knowing this place. In this place there is a permanent sequence of amino acids, that is, a sequence of amino acids that does not change from generation to generation. Why is that place special? This permanent sequence is the "consensus" sequence. Proteases are enzymes that divide proteins. However, the enzymes do not cut the protein chain everywhere, but rather the places where there are certain amino acid sequences. These sequences are called consensus.
Each type of protease only knows a sequence of "consensus". The sequence in V3 can only know some special proteases of the human being and one of them is the receiving molecule called CD26 that is located on the surface of the T4 lymphocyte. The Pasteur Institute team showed that to enter the hostel, HIV needs CD26 protein activity. In its defect there is no infection. What's more, monofuntional antibodies made against CD26 (which can only be fused with CD26) were able to prevent HIV from entering any type of cell. In addition, if cells that do not normally contain CD4 or CD26 cause the production of these molecules, scientists in this group claimed that any type of cell was sensitive to HIV infection.
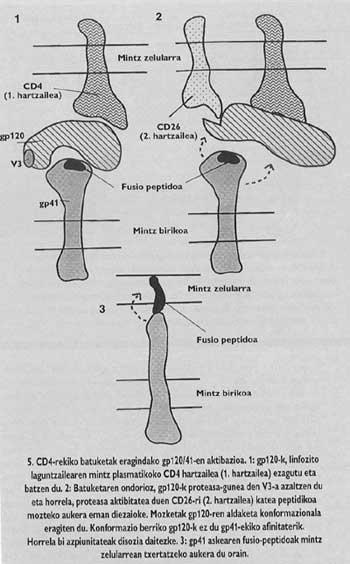
That would, therefore, be the mysterious factor we have mentioned. According to this finding, in the second step of the activation mechanism of the GP41 currently proposed, the CD26 would cut the protein chain at the V3 site of the GP120 which was attached to CD4. The cut would mean a change in the shape of GP120, which would mean a decrease in affinity with GP41. The loss of affinity would cause the release of the gp41 around the membrane of the target cell. This ensures the correct insertion of the fusion peptide.
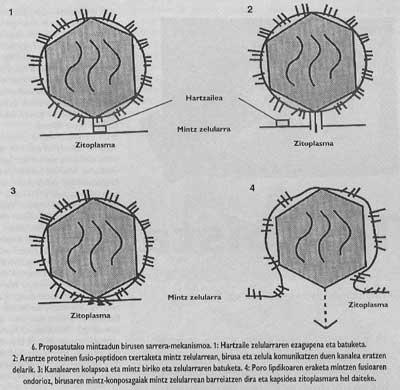
The general scheme of the entry remains uncompleted. Although we know that binding to the receptor and the introduction of fusion peptides are necessary steps, we still do not know how fusion is performed between the virus and the target cell membranes. However, the experimental data we have at this time indicate that the inspecific mechanism (Figure 6) would be the following: once the fusion peptides were inserted, the virus of the spine that has produced the fusion forms a channel that can communicate the cell (this step can say that it has a great experimental base) and later, thanks to a mechanism still not clear, the channel suffers a collapse and, finally, the membrane of the virus and the lipid. Once the pore is opened, the cathode of the virus can reach the cytomopiate cell.
We still do not know to what extent these general processes will be useful to inhibit virus entry, but we can work on possible ways to carry out this hypothetical inhibition. Likewise, as the details of the basic mechanisms of the process are clarified, the possibility of finding solutions will be increasing. In fact, trying to inhibit the process of entry described is not a simple occurrence, but a reality that is already being put into practice today. In order to curb HIV infection, soluble fractions of the CD4 receptor are already being used as the basis of a therapy.
Researchers believe that virions will join these fractions and will be inactivated. As mentioned above, several synthetic vaccines based on GP120 try to wait for antibodies against the immune sites of this peptide to be able to stop HIV infection. In vitro studies have shown that fusion peptides obtained through chemical synthesis can prevent the entry of HIV. It is not yet known what may be the cause of this inhibition, but it is possible that these free peptides cause distortions in the structure of the channels constructed by spiny proteins. In the experiments carried out "in vitro" it has been observed that several synthetic peptides with sequences of operation of several V3 sequences are capable of inhibiting the entry of synthetic peptides.
With the help of lettuce, these peptides can be able to stop the collection process of CD26. With the aim of treating influenza infections, drugs capable of inhibiting the release of hemaglutin fusion peptide are being designed. These drugs would join the HA2 subunit, near the location area of the peptide, and as a plug, would make it difficult to move the fusion peptide. There are agents capable of avoiding the formation of lipid pores that can be used to hinder the fusion of the membranes of the virus and the cell.
Research on the entry of the virus has just begun. It can be thought that in a continuous scientific essay many turning points will appear. At the time nature authorizes us, it is time to design an antiviral therapy that can be used in people. This therapy could be based on the inhibition of the input process. Until that moment arrives, what is clear today is that in the field of viral infection, acting in a single aspect of research as the vaccine does not go to possible points of tragedy. Efforts to understand the basic molecular mechanisms involved in viral introduction will also have a major influence on the search for possible therapies.

- all a virus composed of a Nuclear Acid (DNA and RNA) and a protein cover.
- synthesis of two other molecules using as a model a replication DNA molecule. In some cases, make copies of the virus itself.
- genome Set of genes characteristic of a species. Unlike the Eucharist, in the prokaryotes it is organized on a single chromosome.
- escorias Set of viruses with the same genetic and pathogenic characteristics.
- substance that produces antibodies after its introduction in the Antigen Organism.
- A substance (immunoglobulin) produced by the organism after the introduction of the antibody, which participates in the mechanism of immunity.
- Retrovirus Virus containing RNA as genetic material, many of these types of viruses cause cancer and of this type is also the cause of AIDS.
- immunity obtained by lymphocyte cell immunity (white blood cells) and macrophages.
- glicoprotein In its composition, the heteroprotein or complex protein that contains, in addition to amino acids, a sugar molecule.
- immunogenic Responsible immune.
- endocity Assimilation of something extracellular through the process called endocytosis by a cell.
- endosoma Non-differentiated component of the cell nucleus.
- Organic molecule of carboxyl amino acids and amines groups. Some amino acids are components of proteins.
- Peptide fusion Peptide that launches the process of making a membrane of virus and a cell membrane.
- Molecule in which is produced the binding of a limited number of peptides Amino acids.
- monomer Molecule capable of reacting with its equals.
- monoclonal produced from the cell clone itself.
- Three-dimensional arrangement of other atoms or radicals attached to a forming atom.

Gai honi buruzko eduki gehiago
Elhuyarrek garatutako teknologia