Live pharmacies

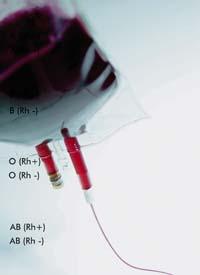
A year ago, the European Medicines Agency (EMEA) authorized the first time worldwide commercialization of a drug produced by a transgenic animal. Specifically, it authorized the use of the ATryn protein produced in milk by genetically modified goat. This protein, produced by GTC Biotherapeutics, is a human antitronbin, anticoagulant. Prevents the formation of coáginas in patients with deficiency of this protein.
Months earlier, the agency did not authorize this antitronbin because it was tested in clinical trials in very few patients. But the lack of antitrobine is a rare disease, only one in 3,000 and 5,000 people suffer, so it is not easy to get many volunteer patients. However, it was subsequently decided that the evidence submitted by GTC was sufficient. Among the volunteers were also pregnant women, and experts showed that antitronbine is totally safe and has the same efficacy in both non-pregnant and pregnant women.
In addition to the GTC company, other companies generating therapeutic substances in transgenic animals took the news with hope. For a long time they expected to open the market doors and soon saw the conclusion of the step taken by SAME: GTC's market value grew by more than 20% and that of its close competition, the Pharming company, grew by almost 10%.
In addition, two weeks after its authorization in ATry, SAME authorized the commercialization of an anti-inflammatory drug produced by Pharming through transgenic rabbits. It was thought that behind this others were going to go.
It is not so. And it is that they have to guarantee that the topics created in transgenic animals are safe and for this they must overcome a lot of tests. In particular, they should consider four points: allergic responses, immune system response, autoimmune response, and infections, including those caused by prions. To ensure and demonstrate that they are safe from all parties, they must use a very advanced technique and present numerous tests. And for this it takes a lot of time and money.
Very special milks
Ten years ago, however, the drawbacks were not known and many researchers expected much in this field. In 1996 the Roslin Institute presented Dolly, the first cloned animal of an adult cell. A year later, in the same institute, two other cloned sheep, Molly and Polly, were born. They did not resemble Dolly much, but were characterized by having a human gene in their genome.
This gene encodes the protein called factor IX. It is a blood clotting protein that is used to treat hemophilia, which is what they lack in the blood. But the only source to obtain factor IX is the blood plasma of human beings and for it they created Molly and Polly to create a flock of sheep that would contribute factor IX in milk. This would represent an important market for this protein.
Therefore, to produce biological molecules such as proteins, antibodies, genetic engineering looked like a great opportunity. Until then, cell cultures of microorganisms, yeasts or animals or people were used, and that is the main route. However, it is a difficult and expensive method, in which the molecules produced in cultures require complex changes to be used in therapy, obtaining also small amounts. For this reason, they considered that the transformation of animals into drug producers could cover the product.
Therefore, they have tried to do so. Molly and Polly did not meet their goal, but researchers have continued to work with cows, sheep and goats. In fact, these animals are used to produce milk, and if, thanks to genetic engineering, these and their descendants manage to form human molecules in milk, we only have to isolate proteins from milk.

On this path, the work carried out by the company GTC with goat has paid off and it is estimated that obtaining antitronbine by transgenic goat is between 30 and 100 times cheaper than production in cell cultures.
Cows produce more milk than goats, so they were selected by Advanced Cell Technology. In the milk of transgenic cows it was intended to obtain the protein seroalbumin of the human blood. Seroalbumin is used to increase blood volume, for example in patients with traumatic injuries. It was considered that cow's milk could be an unbeatable source, since a single cow can provide more than 8,000 liters of milk a year, which could mean between 40 and 80 kilos of protein.
However, this field has not been the expected development. Many projects have been left on the road and, although at present the market of these molecules for therapy moves 24.500 million euros, the products produced from genetically modified animals continue to occupy a very small part.
Eggs and plants
However, researchers do not give up and now they also want to produce human proteins of medical utility in eggs. The method is similar to that used in ruminants. Like ruminants in milk, genetically modified chickens produce therapeutic protein in eggs.

The cultivation of transgenic hens has certain advantages over cattle. They are cheap, productive (each chicken can lay 300 eggs a year) and the intergenerational distance is small. This facilitates the adaptation of production to demand.
The Roslin Institute, in collaboration with Viragen and Oxford Biomedica, has managed to produce two therapeutic proteins in the clear of transgenic chicken eggs. One of them is the Mini-R24 antibody, allegedly useful against malignant melanoma, and the other human interferon b-1, used to treat multiple sclerosis and other diseases. The research, published this year in the scientific journal PNAS, aims to continue investigating to improve the results.
However, in addition to chickens, ruminants have other competitors: the plants. Researchers have been looking for years so that plants have characteristics that they want to genetically transform, including the ability to produce human proteins and vaccines.
Transgenic plants are easier to grow than animals and also have a lower risk of virus or prion infection. On the contrary, they cause more allergies and, above all, they have a high risk of spreading to the environment. In fact, for many people this is one of the biggest reasons to be against GM crops. Therefore, manufacturers are required to take drastic measures to avoid this risk.
For example, researchers at the US Purdue University grow transgenic plants in a mine located in Indiana. In this way, it is guaranteed that the external plants will not be affected. They research with maize, tobacco, alfalfa and soybeans, among others, to create antibodies, insulin and vaccines.

There are numerous studies of transgenic plants capable of producing this type of molecules, and recently the U.S. Department of Agriculture. has received the authorization to grow rice that produces proteins found in the female milk. Transgenic rice contains lysozima, laktoferrin and human seroalbumin. Lysozyme and laktoferrin have antibacterial, viral and fungal properties, and with rice, researchers want to make a drink to treat diarrhea and anemia.
Despite the problems, genetically modified animals and plants are becoming a source of therapeutic molecules. However, they are still in their beginnings, while the effectiveness of the systems of generation of these molecules in cell cultures is increasing. Time will tell what is the best way, for now researchers do not want to close the door.

Buletina
Bidali zure helbide elektronikoa eta jaso asteroko buletina zure sarrera-ontzian











