If pregnancy heals the heart?
2018/03/01 Redondo Angulo, Ibon - Biomedikuntzan doktorea Iturria: Elhuyar aldizkaria
The heart of the mammals has as its only function to pump blood through the circulatory apparatus to bring to the heart and the rest of the organs nutrients and oxygen. The task is unique, but not slow. The situations that occur throughout animal life present very varied energetic demands that the heart must fulfill.
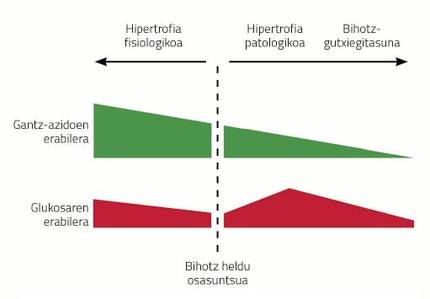
To meet these higher energy demands, the heart has to work more and that has a consequence: more stress on the walls of the heart. If this increase persists over a period of time, the heart can initiate a mechanism to reduce ventricular stress and maintain or increase the pumping capacity. Unfortunately the name of the mechanism is known: cardiac hypertrophy.
Cardiac hypertrophy
Cardiac hypertrophy is a consequence of diseases such as hypertension, infarction caused by ischemia of the coronary arteries or obesity. This type of hypertrophies are called pathological hypertrophies and have certain characteristics. For example, the metabolism of the heart changes radically with the hypertrophy of the heart. Unlike the brain, the heart mainly uses fatty acids (60-70%) as a source of energy and the rest gets it from glucose metabolism. When the heart becomes sick, the use of energy sources changes radically; glucose becomes the main source of energy in the hypertrophied heart and fatty acids are discarded. If the chronic stress that receives the hypertrophied heart does not disappear, the heart increases even more, more fibrosis appears and inflammation occurs decreasing the pumping capacity. In addition, as the disease progresses, the heart stops using glucose, characteristic of heart failure.
Cardiovascular diseases are the most frequent in today's society and many of them have the symptom of an increase in the heart. Heart hypertrophy is often transformed into heart failure which, unfortunately, still has no remedy. Therefore, heart failure is one of the most common causes of death in industrialized societies.
Numerous biomedical studies in this field are being tested to discover and understand the molecular mechanisms by which cardiac hypertrophy becomes heart failure. In this way, new therapeutic episodes could be developed to avoid hypertrophy of the heart or for the hypertrophied heart to return to its healthy state, all to avoid heart failure. But where to start, how to make a hypertrophied organ to return to its normal anatomical function and state? For this we should not look very far or in depth, females can have response, pregnant females.
Cardiac hypertrophy by pregnancy
Gestation is a complex, even biological process. The fertilization begins numerous profound changes in the organism of a female. All these changes will allow a new member of a species to emerge from the union of the two cells.
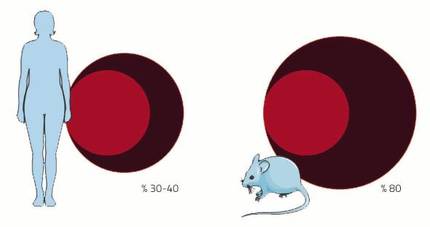
The cardiovascular system is one of the systems that will undergo many changes during pregnancy. The cause is the placenta. As if it were little the birth of a new child, gestation has another wonderful fact: creating a new organ within an adult body. This new organ needs, like the rest, a supply of blood. For this purpose, the arterial and venous network will continue until the diet to supply blood to the placenta. In addition, the blood volume will be increased to fill the new prolonged circulation apparatus. In women the volume of blood increases between 30-40%, almost double that in the mouse 80%. And it is that, as in every pregnancy the mice have as many pleasing as young, their blood demand is greater.
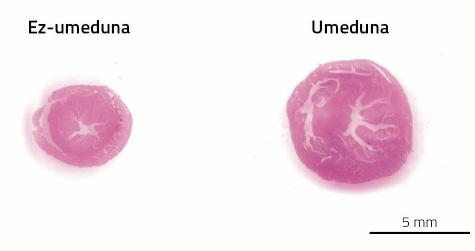
The problem is that the heart has to pump more blood from a longer system of pipelines of arteries and veins. As discussed above, gestation is one of the situations of animal life in which the heart has to work more. As a result, pregnant females develop cardiac hypertrophy. This type of hypertrophy, on the other hand, is called beneficial or physiological. In this case, the heart does not express fibrosity, and the metabolism does not select glucose but the opposite is based more on fatty acids (see figure 3).
After childbirth, like the placenta, the physiological changes derived from pregnancy disappear. Also hypertrophy of the heart. In the case of mice, between 7 and 14 days after childbirth, the heart is able to recover its original size; in women the process extends for one year.
There is the question. How does the heart of a hypertrophy pregnant female exclude? Why is it not possible to restore cardiac hypertrophy that occurs in various diseases? What can we learn from the beneficial hypertrophy of pregnancy to cure pathological hypertrophies? In response to this, the research carried out in our laboratory has described 2 factors related to gestational hypertrophy:
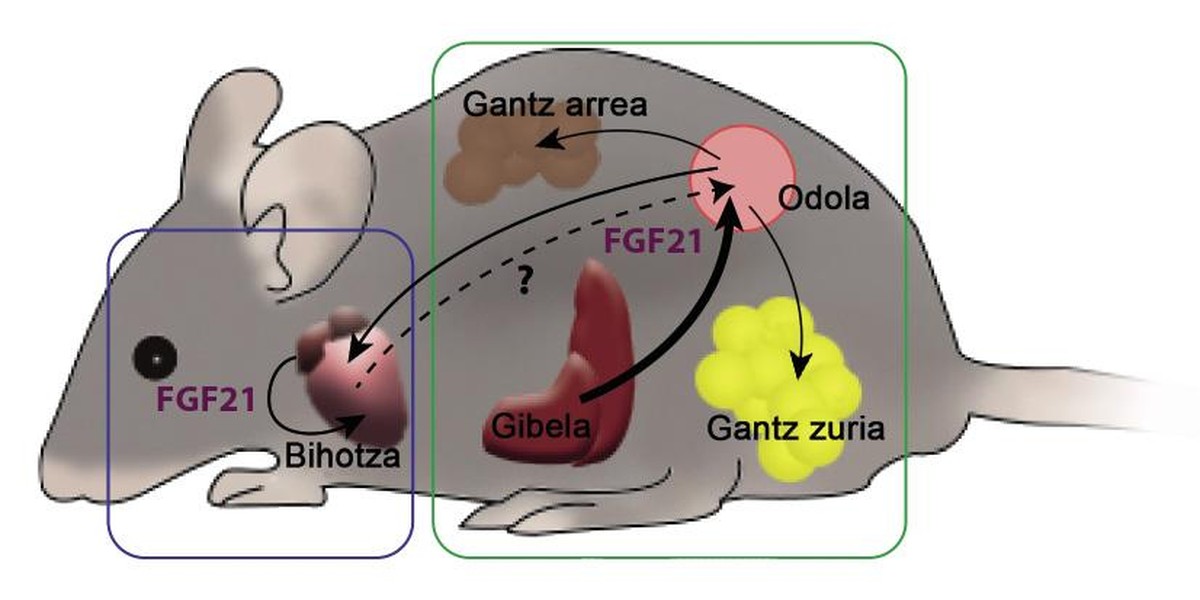
? The first factor is FGF21, growth factor of fibroblasts 21. In 2000, a group of Japanese scientists discovered this factor as a hormone. The FGF21 factor is mainly generated in the liver, then travels through the blood to reach its target tissues, where it produces various effects. One of them is brown adipose tissue or brown fat. This hormone promotes the consumption of fatty acids. In general, FGF21 is a factor capable of stimulating metabolism, so perhaps the hormone has also been presented as a fat smoker. Some laboratories also work to use them as an anti-diabetes tool. Diabetic rodents treated with FGF21 had better levels of glucose or glycemias, while recovering the sensitivity to lost insulin and reducing the weight and triglycerides in the blood.
The relationship between FGF21 and the heart has been unknown until 5 years ago. Then we published how the FGF21 was beneficial also to the heart. Mice were generated a pathological hypertrophy of the heart by a drug. In these hearts appeared fibrosis, inflammation and the restricted indication of the genes that govern the consumption of fatty acids. Another mouse group, in addition to the drug, was inserted FGF21, which caused a lower cardiac hypertrophy. The degree of inflammation and expression of the genes had also improved compared to animals who only received the drug. In addition, among the discoveries, we show that the heart has the capacity to produce FGF21, which would allow the heart to influence it the FGF21 which he himself has created.
During pregnancy, a higher physiological expression of FGF21 occurs. The liver and also the heart generate a factor, so the degree of FGF21 measurable in the blood of a pregnant female is much higher than that of a non-pregnant female. In addition, attending to the consumption of fatty acids of the heart, during pregnancy is much greater, which means a beneficial hypertrophy. In another group of experiments, we worked with mice unable to create FGF21, so that, despite being awake, females could not increase the blood level of the factor. From this it was deduced that the ability to use fatty acids was reduced. The FGF21, therefore, is necessary to carry out the work overload that gestation demands to the heart, since the heart needs FGF21 to consume more fatty acids.
? The second factor described is a transcription factor whose function is to channel the expression of other genes. When analyzing pregnant females, we highlight that the presence of this second factor was only in the heart. Being so specific this overexpression of the heart, it seemed to us that we had to have a reason behind. Factor C/EBPb is a factor closely related to immune response and inflammation, as it is key to macrophage activation. Macrophages are cells that intervene in the inflammatory process and can be of two types: proinflammatory (type M1), which will initiate the process and anti-inflammatory (type M2), which will resist the process.
As has already been indicated, in the case of pathological hypertrophies, fibrosis and inflammation can be observed in the hearts, not so in physiological hypertrophies. In the case of gestation it was described that, being beneficial, the presence of macrophages type M2 was greater than that of type M1. If pregnant females are removed half of the capacity to use C/NFU, the opposite occurs in the hearts: The macrophages type M1 predominate. The heart needs C/EBPb to activate macrophages M2 and combat inflammation.
The heart has, therefore, mechanisms and ways to make beneficial a situation that should be pathological and allow a higher workload than normal, as well as the subsequent elimination of hypertrophy. What's more, these fertilization mechanisms, and the others that are to be discovered, are capable of protecting the heart. A couple of studies reveal how the hearts of rodents children were more resistant to a drug that produces fibrosis, and how the damage caused after an infarction was lower in children.
It should be noted that most of the biomedical research carried out with rats and mice is carried out with male animals, either because it has always been done so or because the females must be kept to have offspring. In spite of this, in this example, females have been the key to the advance towards hypertrophy and heart failure, since they are the only ones that have contributed the pregnancy.
Bibliography Bibliography Bibliography
Work presented at the CAF-Elhuyar Awards.

Gai honi buruzko eduki gehiago
Elhuyarrek garatutako teknologia