Prions, guardian of memory
2017/03/01 Agirre Ruiz de Arkaute, Aitziber - Elhuyar Zientzia Iturria: Elhuyar aldizkaria
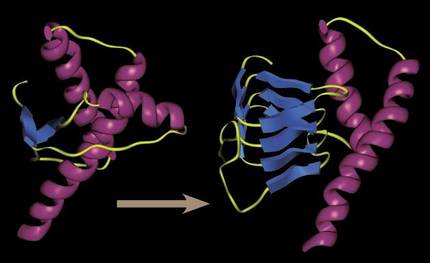
30 years ago it was unimaginable to talk about an infectious organic system capable of “reproducing” without DNA or RNA. All infectious systems had genetic structures to increase infection and virus proliferation, the simplest yet known infectious agents. But prions showed that it was possible: simple, wrongly folded proteins were able to infect the surrounding proteins with their incorrect folding. By contact, no need to reproduce.
They managed to alter the three-dimensional shape of the functional proteins that accompanied them, and being a three-dimensional form generated of a deengorable character, prions tended to congregate creating increasingly large aggregates and hindering cellular processes.
The diseases caused by prions triggered alarm from the beginning, in the crisis of mad cows, when the scientific community was speechless about this new form of infection. The mere fact of being prions was exciting from the biological point of view, it could not be denied, but at the same time it was terrifying. They didn't know where to tackle prion infections. In addition, it has been observed that they may be at the base of many neurodegenerative diseases, such as Alzheimer's and Parkinson's.
However, after many studies, the friendly face of prions has begun to emerge: non-pathogenic prions, creators of aggregates and with specific tasks in our body.
Long-term memory at the hands of prions
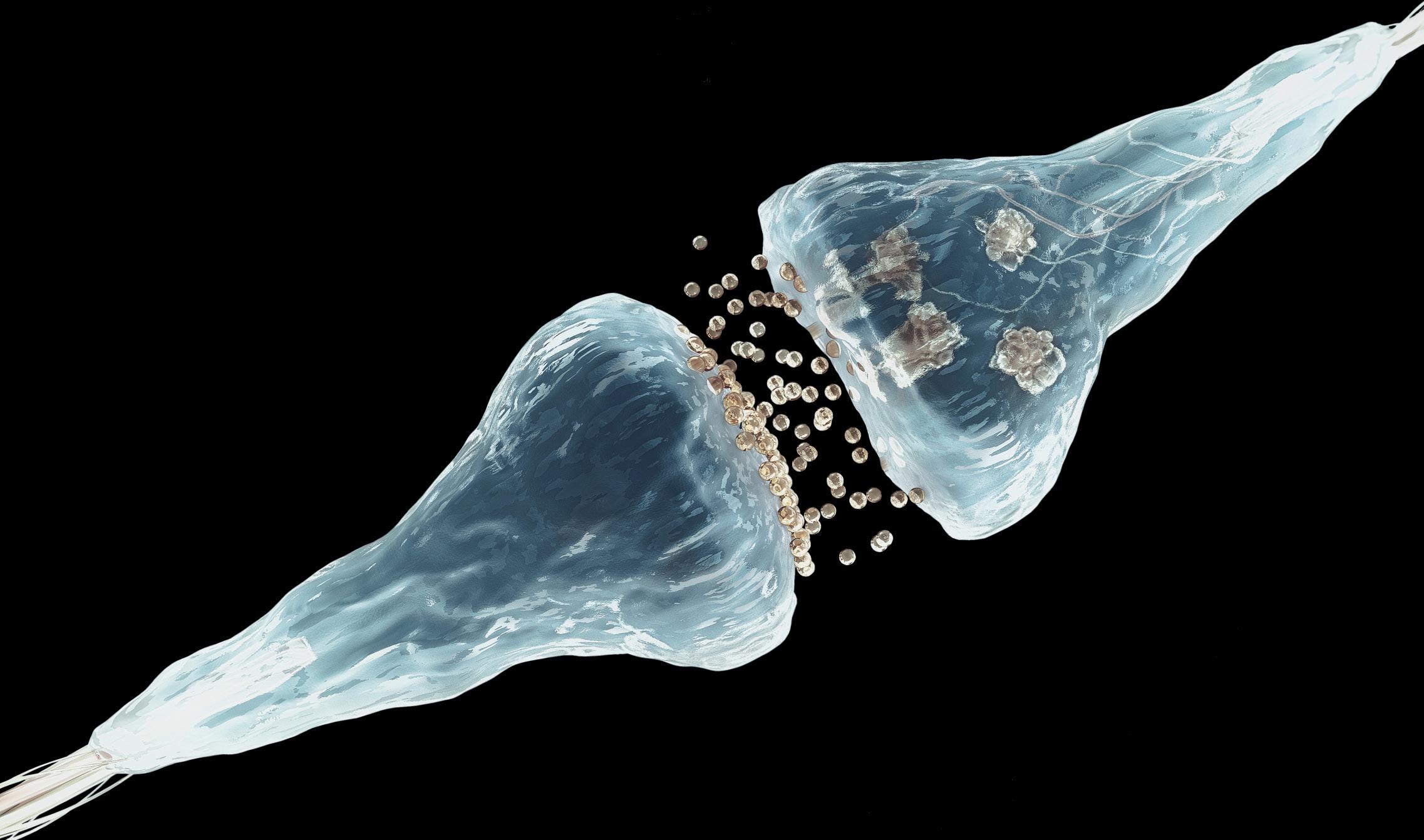
If we look at the brain, we will see a billion neurons, each with many synapses. In these synapses memory is fixed, thanks to them we chemically store memories. Francis Crick, one of the discoverers of the DNA structure, however, expressed 20 years ago a concern: How can memories stay much longer than proteins remain in living cells? If the synapse proteins last two months, how can a memory be chemically maintained for years? How can a synapse be stabilized forever?
Crick foresaw the presence of a protein that is added in some way in this process. Being added, it would have no ability to move, and that could be the way to stabilize a certain synapse.
Then they saw that prions appeared in the synapses and began experimenting to train the mice to cross a labyrinth over and over again, until that experience consolidates as a long-term memory. Through genetic engineering, they silenced the CPEB prion gene, typical of neuronal simapses, and the results were surprising: two weeks after the consolidation of memory had already disappeared.
The same have been seen in the vinegar flies. If a female does not show a copulation position with respect to a particular male, that male will remember it and will not try again, even if it has been a long time. By mutating the Orb2 protein that acts as prion, from a single day, memories become unstable; after three days, they have completely disappeared. The male will try to cover it again.
From there they have seen that prions serve to strengthen and stabilize neuronal synapses, which allows to maintain long-term memory. Prions seem ideal for this work. In fact, they create very stable polymer structures over time and their conversion is self-sufficient: when a prion changes its bending, the surrounding proteins will continue to automatically change its folding. This process of prion infection solves the problem of Crick, since, beyond the initial stimulus of the neuron, they will continue to create aggregated chains without further stimuli. This will keep the synapse constantly and therefore the memory.
But we do not keep all memories forever. The neuron, with strict regulation, separates the stabilized neural circuits and which do not. When it receives the electric pulse, in a complex biochemical dance that only occurs in that synapse, it activates the bending change and activation of the prion with the placement of a group of phosphates. From there, as long as the prion aggregates are maintained, long-term memories will remain. They are the biochemical basis of memory.

Their own responsibility as a memory system turns prions into pathogens of neurons and memory. This could mean their involvement in Alzheimer's and Parkinson's diseases.
Flowering plants
Mammals are not the only ones who take advantage of the work of prions. For example, genetics from the Massachusetts Institute of Technology (MIT) have found in plants about 500 proteins with prion characteristics. Many of them have functions related to achievement.
Plants develop memory to respond to environmental conditions. They record droughts, heating, cooling and pathogens previously suffered. The memory of hibernation, for example, develops after feeling cold for a long time. Thanks to this memory, the plants remember the winters that have passed and regulate the moment of flowering. According to MIT researchers, prions are essential to record long-term environmental conditions and regulate the flowering process of futures. In some way, in the offspring also help to preserve the memory of the cold, from generation to generation, without the need to involve the genes. Thanks to them, plants that have not yet suffered cold have this memory. According to MIT researchers, prions have provided the key to understanding protein-based molecular memory.
Also bacteria
Until now, bacteria were the only ones that showed no signs of prions among living beings, but recent studies have shown that bacteria also have them. 60,000 bacterial genomes have been analyzed in search of genetic sequences similar to yeast prions and have seen that a protein sequence called Rho could be a good candidate. Thus, it has been proven that the Rho protein of the bacterium Clostridium bolutilum is injected into the bacteria Escherichia coli and behaves like prions.
Rho is a component that regulates the expression and activity of many genes. By injecting the normal version of the protein Rho they saw that it silenced the genetic activity of Escherichia coli and that by injecting the prion version many genes were activated. In view of this, researchers believe that in the case of bacteria, by regulating genes, prions can help adapt to abrupt changes in environmental conditions. This would allow rapid response to changes in conditions, such as the presence of an antibiotic.
In addition, because prions are hereditary, it may be thought that they will allow bacteria to inherit these characteristics without the need for genetic mutations.
Because of their presence in bacteria, prions seem to be much more widespread in nature than previously thought, and appeared before eukaryotes emerged more than 2.3 billion years ago. But where do these mysterious proteins come from?
Prions have never been among those who go unnoticed. They are used to giving surprises. However, we do not know what other capabilities will give them their special character. Researchers say that they have already begun to understand them and that they will almost necessarily bring more surprises.

Gai honi buruzko eduki gehiago
Elhuyarrek garatutako teknologia