Nobel Prize in Chemistry 2010 for facilitating the synthesis of large organic molecules
2010/10/06 Lakar Iraizoz, Oihane - Elhuyar Zientzia

Simple carbon bonds fundamental to organic chemistry are formed in a chain of reaction called cross coupling. It is therefore a very useful technique for the synthesis of complex organic molecules. Reactions occur in simple laboratory conditions and joints are performed with great efficiency and precision.
When it comes to forming complex organic molecules, chemicals normally use smaller molecules that will be their components. They just have to attach small molecules to get larger molecules. However, these molecules are usually stable and it is not enough to put them together for this bond to occur.
That's where palladium becomes important. The two binding molecules first bind to palladium. In this way, the two carbon atoms that must form a union with each other are closer. In addition, its relationship with palladium increases the tendency to interconnect both carbons. Once the carbon-carbon bond is produced they are easily released from palladium and it is ready to participate in another cross link. That is why it is said to be a catalyst because although it allows the reaction to occur it is not one of the components.
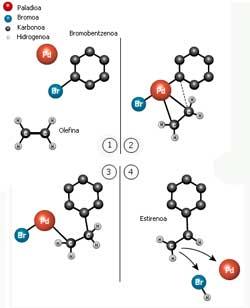
From 1 to 4 representing the reaction described by Hecke. Bromobenzene and olefin bind first to palladium and then form a carbon-carbon bond between them (Photo: Wegune official of the Nobel Prize).
Three winners, three contributions
In 1968 Richard Heck published his first articles on the carbon links obtained by palladium as an intermediary. He joined a carbon ring with an olefin (a two-carbon molecule bound by a double bond) by palladium. Styrene, the most important component of plastic polystyrene, is obtained from this reaction.
Then came the contributions of Negishi and Suzuke. The atom associated with the carbons involved in the reaction has a great influence on the reaction: some are more selective, others modify the conditions necessary for the reaction to occur, etc. Thus, the paths addressed by these two researchers have allowed cross-link reactions catalyzed by palladium to occur more easily and accurately.
In 1977 Negishi discovered that if one of the two carbons contained a zinc atom, the reaction was more effective and highly selective, and that reaction conditions could be easily formed.
Moreover, in 1979 Suzuki proposed the carbons associated with boron to carry out this reaction. Boron is less toxic than zinc, it is also very selective and can be associated with many functional groups.
A tool that has improved our way of life
These pathways of synthesis of complex organic molecules have allowed the advancement of organic chemistry and its associated practical applications. They are used in industry to produce various compounds and products. Such industrial use is because reactions do not require special conditions.
It is used to synthesize compounds found in nature, for example. Taxol, for example, is a very effective antitumor component and was found in the Pacific vagina ( Taxus brevifolia). The cross coupling catalyzed by palladium allowed synthesizing in the laboratory. Through this reaction, variants of natural antibiotics have been developed that have also been able to affect resistant bacteria.

Gai honi buruzko eduki gehiago
Elhuyarrek garatutako teknologia





