Chemical element 110,
2003/07/14 Kortabitarte Egiguren, Irati - Elhuyar Zientzia
Therefore, the finding of element 110 resulting from the collision or fusion reaction between certain lead and nickel isotopes is noteworthy. This element, also known as darmstadtio (Ds), first appeared in a German laboratory in 1994. It was soon discovered in the U.S. Berkeley laboratory and other laboratories in Russia. In both cases a total of seven isotopes of the same element were found and the lighter and heavier were 157 and 171 neutrons respectively.
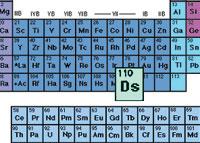
Darmstadio, in the periodic table, is found in the same group as nickel, palladium and platinum, specifically in group 10. However, unlike these lighter atoms, darmstadio rapidly disintegrates into lighter atoms and emits alpha particles.
The discovery of this new element leaves aside the tension
generated around element 118. In fact, scientists discovered this last element of
the alpha particles released in the disintegration of the giant atom, resulting from the collision between lead atoms and crypton. Although two years later they tried to repeat the experiment, they found no trace of this element. And that, of course, only caused suspicions and scandals.
On this occasion it can be said that there is this chemical element 110 of the Periodic Table, since repeated trials have given good results.
More information:
Physicsweb

Gai honi buruzko eduki gehiago
Elhuyarrek garatutako teknologia