[Microbiota and metabolic disease]
The relationship between microbiota and metabolism has opened new doors in the world of research in recent decades. Our organism is made up of billions of unimaginable microorganisms, whose balanced composition plays a vital role in health. Obesity, diabetes and/or metabolic syndrome are diseases which may be caused by imbalances in said composition, e.g.
in 1914, the philosopher Ortega y Gasset pronounced the famous phrase “I am I and my circumstances.” In this case, the phrase “I am me and my microbiota” sounds better to us.
The microbiota is made up of all the microorganisms that live in our body. It is mainly composed of bacteria, but also includes fungi, yeasts and viruses, among others [1]. How about viruses? Calmly, the relationship between our organism and these microorganisms is generally symbiotic, that is, this relationship is beneficial for both parties. In fact, they maintain the normal physiological state of the body in a dynamic equilibrium [2] and participate in immunity [3].
We find the microbiota in our body wherever it is in contact with the external environment: in the intestines (the famous intestinal flora), in the mouth, skin and vagina [1]. In total, it contains 38 billion microorganisms, more than the human cells of the body (30 billion) [4]. In other words, microbial cells are more abundant in our body than human cells.
The intestinal microbiota or intestinal flora is the most important, even the most variable, microbiota. It has the following three main characteristics: complexity, dynamicity and heterogeneity [2].
It is complex because it is made up of many different microorganisms. However, as with surnames, there are some groups of bacteria that are very numerous: Firmicutes, Bacteroidetes, Actinomycetes, and Proteus [2]. It is dynamic because it can vary depending on the diet or lifestyle. Finally, it is heterogeneous because the composition of the intestinal flora differs from one part of the intestine to another.
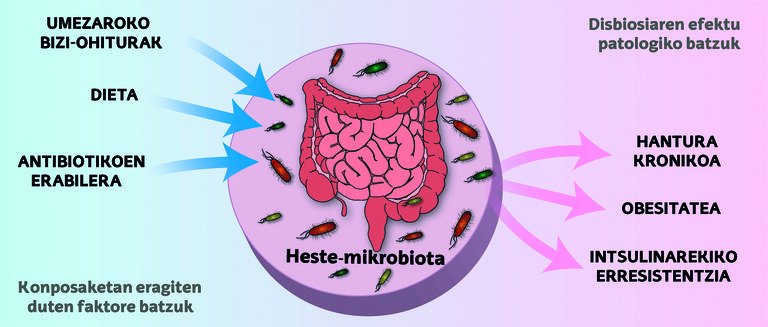
As mentioned, within our body there is a tumultuous world of microbes, and these small organisms play a vital role in our health. Interestingly, this microbial community begins to emerge before birth, as the mother transmits it to the fetus [5].
However, it continues to develop after birth and is influenced by factors such as the method of delivery, diet (breast milk or formula), hygiene and the use of antibiotics. It should be noted that the first three years of life are essential for the formation of a stable microbiota similar to that of an adult; subsequently, this microbio-community has a significant impact on both our immune and neurological systems [5].
However, throughout life, the intestinal ecosystem can undergo changes that affect the delicate balance between microbes and our body, a condition that is known as dysbiosis. These alterations can lead to problems such as inflammation, metabolic disorders and/or insulin resistance, which increase the risk of metabolic diseases (Figure 1) [5].
The community of microorganisms that live in our intestines has had a growing scientific interest in recent decades. As the understanding of this biological network deepens, illustrative connections have been found between the composition of the microbiota and various health conditions, including diabetes, obesity, and metabolic syndrome.
The presence of obesity
We all know what obesity is, but how does the World Health Organization define it? Obesity is an excessive accumulation of fat that poses a health risk [6].
Where does that fat come from? Well, after all, it is the result of a positive energy balance [7]. That is, it occurs when the calories we take from our diet exceed what our body needs to perform its functions.
But why doesn't my friend get fat if he doesn't play sports and eat a lot? Obesity, like most diseases, also depends on genes [8]. But lately, it has been observed that the microbiota also has an effect.
One study compared the microbiota of obese mice and lean mice [9]. Polysaccharides that mammals cannot digest and absorb (i.e., large and complex sugar molecules) can be digested by the microbiota of obese mice because it contains a variety of scissors for this purpose. These scissors, called enzymes, allow polysaccharides to be cut and converted into simple sugars, which can be absorbed by them.
Therefore, in summary, obese mice, thanks to their microbiota, make better use of the calories consumed. In fact, the feces of obese mice contain fewer calories. For this reason, even though obese mice and lean mice are fed the same way, the energy balance of obese mice will be more positive and they will accumulate fat. Moreover, if the microbiota of obese mice is transplanted into lean mice, the lean mice begin to accumulate fat.
All of this, of course, can be extrapolated to humans. And yes, although it may seem disgusting, the manipulation of the microbiota can become an important therapeutic strategy to regulate the energy balance in obese people [7].
[Diabetes and metabolic syndrome]
We have already seen what obesity is, but how can diabetes and metabolic syndrome be defined? Metabolic syndrome is a group of metabolic abnormalities that refer to the coexistence of several risk factors for the development of cardiovascular disease. Among these metabolic abnormalities, insulin resistance can be found, among others [10]. Diabetes is a chronic disease that occurs when the area does not secrete enough insulin and/or the body does not use the produced insulin effectively [11]. What are we talking about when we talk about insulin? Insulin is a hormone that regulates the concentration of glucose in the blood, blood glucose. When the concentration of glucose in the blood is very high, the hormone insulin is released and the concentration is reduced and the correct regulation is maintained [12].
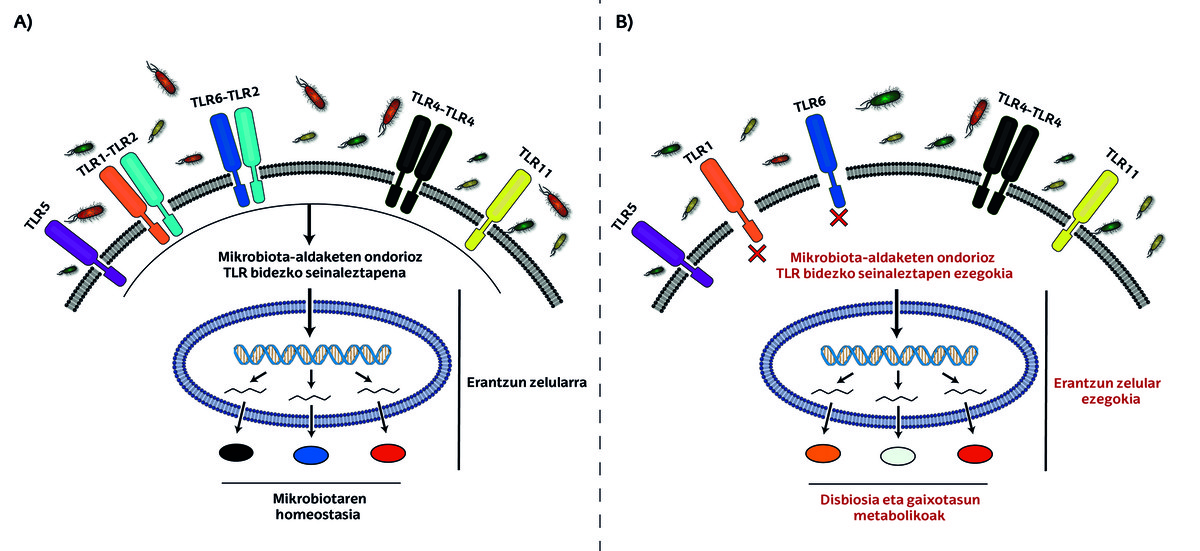
It's clear, isn't it? If so, to move forward in this story, it’s time to introduce participants called TLRs!
TLRs or Toll-Like Receptors are microscopic antennas that detect signals in our body. These receptors are present in the cells of the intestinal epithelium and regulate the colonization of bacteria, that is, they are responsible for maintaining the amounts of different bacteria at the appropriate level [13].
Although there are numerous subgroups within the TLR family, TLR2 is particularly important in diabetes. It has been observed that there are fewer bacteria of the genus Bifidobacterium in the intestinal flora of mice without TLR2 receptors. These bifidobacteria act as patches in the intestine, and their deficiency results in an increase in intestinal permeability. This will cause bacterial toxins to pass through the intestinal epithelium and into the circulation, causing inflammation and increasing the risk of diabetes mellitus disease. This mechanism gives us two treatment options. In one aspect, antibiotics that attack bacteria of other genera can be used to enhance the proliferation of bacteria of the genus Bifidobacterium. On the other hand, the microbiota of healthy mice can be transplanted into the intestinal flora of TLR2-deficient mice [7].
Likewise, an imbalance in some intestinal bacteria can cause problems that are directly related to insulin resistance and, therefore, diabetes. As mentioned, insulin regulates the concentration of glucose in the blood. If insulin resistance develops, the body's cells will not be able to respond to insulin properly. In principle, the body can maintain blood sugar levels within the normal range by increasing insulin production. But as insulin resistance worsens and the pancreas can no longer produce enough insulin to overcome this resistance, blood glucose levels rise, leading to type II diabetes. Type II diabetes can therefore be understood as hyperglycemia caused by the presence of insulin resistance [14].
Confirming this, associations between the composition of the microbiota and insulin resistance have been found in people with type II diabetes [7]. So the question of type II diabetes has become clear, yes, but what about type I diabetes? The microbiota can also be associated with type I diabetes, an autoimmune disease that occurs early in life. An autoimmune disease is a disorder caused by the immune system itself, which attacks the body's own cells. In this case, type I diabetes selectively destroys some cells in the area and causes insulin deficiency [15]. Studies in mice and rats have shown that some intestinal bacteria affect the incidence of this disease and that the imbalance of TLRs and the altered composition of the microbiota can lead to type I diabetes [7].
Finally, and continuing with the mice, in studies with these animals, it was found that those raised in a germ-free environment (with all TLR receptors at the appropriate level) were protected from insulin resistance, obesity and other conditions, despite the fact that the given diet was rich in fat. In contrast, colonization of these protected mice with the intestinal microbiota of mice deficient in the particular TLR5 receptor rapidly developed metabolic syndrome. This particular receptor recognizes microbial patterns, so the study revealed the relationship of the metabolic syndrome with the microbiota. As if this were not enough, some specific communities of intestinal bacteria associated with metabolic syndrome have also been identified in humans [7].
There is still a lot to investigate about the microbiota. Technical advances have led to a growing understanding of the underlying mechanisms of obesity, diabetes, metabolic syndrome and other diseases, as well as their interrelationships. This opens the door to new opportunities for prevention and treatment in the future, while at the same time it is hoped that the burden of these diseases on health systems can be alleviated...
The bibliography
[1] Hou, Kaijian, Zhuo Xun Wu, Xuan Yu Chen, Jing Quan Wang, Dongya Zhang, Chuanxing Xiao, Dan Zhu, Jagadish B. Koya, Liuya Wei, Jilin Li, and Zhe Sheng Chen. 2022. “Microbiota in Health and Diseases.” Signal Transduction and Targeted Therapy 7(1).
[2] Chen, Yinwei, Jinghua Zhou, and Li Wang. 2021. "Role and Mechanism of Gut Microbiota in Human Disease" Frontiers in Cellular and Infection Microbiology 11.
[3] Belkaid, Yasmine, and Oliver J. It's about Harrison. 2017. “Homeostatic Immunity and the Microbiota.” Immunity 46(4):562–76.
[4] Sender, Ron, Shai Fuchs, and Ron Milo. 2016. "Revised Estimates for the Number of Human and Bacteria Cells in the Body." PLoS Biology 14(8). For the exact: 10.1371/journal.pbio.1002533.
[5] Boulangé, Claire L., By Ana Luisa Neves, Julien Chilloux, Jeremy K. Nicholson, and Marc Emmanuel Dumas. 2016. “Impact of the Gut Microbiota on Inflammation, Obesity, and Metabolic Disease.” Genome Medicine 8(1).
[6] https://www.who.int/health-topics/obesity#tab=tab_1
[7] Hemarajata, Peera, James Versalovic, and Lucero Dra Lau. 2013. “Selected Translations of Clinical Chemistry Human Intestinal Microbiota and Corporate Metabolism: Implicaciones Con La Obesidad y La Diabetes`Sridevi Diabetes`diabetes`Sridevi.” Acta Bioquím Clín Latinoam 47(2):617–45.
[8] Singh, Rajan Kumar, Permendra Kumar, and Kulandaivelu Mahalingam. 2017. “Molecular Genetics of Human Obesity: A Comprehensive Review.” Comptes Rendus - Biologies 340(2):87–108.
[9] Turnbaugh, Peter J., Assisted by Ruth E. Law, by Michael A. By Mahowald, Vincent Magrini, Elaine R. Mardis, and Jeffrey I. It's about Gordon. 2006. “An Obesity-Associated Gut Microbiome with Increased Capacity for Energy Harvest.” Nature 444(7122):1027–31. For the exact: 10.1038/nature05414.
[10] Huang, Paul L. 2009. “A Comprehensive Definition for Metabolic Syndrome.” Disease Models & Mechanisms 2(5–6):231–37. For the exact: 10.1242/dmm.001180.
[11] Darenskaya, M. I'm talking about A., L. I. Kolesnikova, and S. I. Assisted by Kolesnikov. 2021. “Oxidative Stress: Pathogenetic Role in Diabetes Mellitus and Its Therapeutic Approaches to Correction." Bulletin of Experimental Biology and Medicine 171(2):179-89. For the exact: 10.1007/s10517-021-05191-7.
[12] Andrali, Sreenath S., Organized by Megan L. Sampley by Nathan L. Vanderford, and Sabire Özcan. 2008. “Glucose Regulation of Insulin Gene Expression in Pancreatic β-Cells.” Biochemical Journal 415(1):1–10. For the exact: 10.1042/BJ20081029.
[13] Yiu, Jensen H. According to C., By Bernhard Dorweiler, and Connie W. I'm talking about Woo. 2017. “Interaction between Gut Microbiota and Toll-like Receiver: From Immunity to Metabolism.” Journal of Molecular Medicine 95(1):13–20. For the exact: 10.1007/s00109-016-1474-4.
[14] Ta, Azeez. 2020. “Hypomagnesemia and Insulin Resistance: Gaining Better Understanding of the Pathophysiology of Type 2 Diabetes.” Insights Biomed 5(4):12. doi: 10.36648/2572-5610.4.4.76.
[15] Chellappan, Dinesh Kumar, Nandhini S. Sivam, Kai Xiang Teoh, Wai Pan Leong, Tai Zhen Fui, Kien Chooi, Nico Khoo, Fam Jia Yi, Jestin Chellian, Lim Lay Cheng, Rajiv Dahiya, Gaurav Gupta, Gautam Singhvi, Srinivas, Philip Hansai, Michael Kamp and Hansai Kamal. 2018. “Gene Therapy and Type 1 Diabetes Mellitus.” Biomedicine & Pharmacotherapy 108:1188–1200. For the exact: 10.1016/j.biopha.2018.09.138.
Buletina
Bidali zure helbide elektronikoa eta jaso asteroko buletina zure sarrera-ontzian











