Talidomide, 50 years later
2010/03/20 Galarraga Aiestaran, Ana - Elhuyar Zientzia
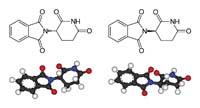
In fact, thalidomide has beneficial effects, hence its use in the late 1950s and early 1960s. Synthesized by Dr. Wilhem Kunz in 1953, it was launched by the German pharmacy company Chemie Grunenthal. Before, the company tested for two years in embryo animals (monkeys, rats, rabbits and dogs). Subsequently, it presented the results to the German government: according to reports, it did not produce any collateral effect either on mothers or on offspring. Thus, the government authorized its use in people and in 1957 Chemie Grunenthal launched it as a sleeping pill.
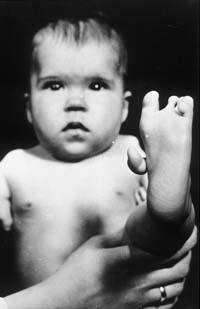
About 50 years ago, 20,000 children were born malformed by thalidomide. So far they have not known how thalidomide affects these consequences Photo: (American Institute of Health)
Born on the Christmas day of 1956, the first child with malformations by thalidomide. Chemie was the daughter of a Grunenthal worker, whose wife took the pills that were distributed free of charge to her at work when she was pregnant.
Then in the countries of sale of thalidomide began to appear more cases: some had totally distorted the muscles of the face, others had short arms or legs, or had no ear, or were blind, others with alterations of the heart, digestive system or kidneys... Experts estimate that in the 7 years that the drug was available to pregnant women there were 20,000 children with these consequences. Of these, 40% died before the first year.
Questioned reports showing that thalidomide was not toxic, a pediatrician at the Hamburg hospital presented 14 cases at a meeting in November 1961. According to the pediatrician, the mothers of these children received thalidomide in the first trimester of pregnancy and the children suffered damage by the action of the drug. Chemie Grunenthal rejected the complaint.
Shortly afterwards, another doctor published in the prestigious medical journal The Lancet an article that showed that thalidomide was guilty. And then yes, Chemie Grunenthal removed him from the market. November 1961. He was retired in Britain in December, in Canada in March 1962 and in Spain in January 1963.
Victims and guilty parties
The trial against Chemie Grunenthal lasted until 1974. The company was acquitted in the criminal case and only had to respond civilly to the victims. He declared his insolvency and paid only 100 million marks; the German government put 150 million more and thereby compensated 2,866 victims. In Britain, Sweden, Canada and Japan, the governments compensated and elsewhere, as in Spain, the victims have not been compensated.
Meanwhile, the victim continues to affect. In fact, it is very effective in treating leprosy, multiple myeloma, and other cancers, and although patients who use it in the United States take steps to prevent pregnancy, in Africa and South America do not.
Now, at the Local Institute of Technology, it has been proven that thalidomide makes it difficult to produce a protein called a cereblon, so the extremities and organs do not develop properly. The next step is to create a substitute for thalidomide without that effect. If they get it before late.
Published in Gara

Gai honi buruzko eduki gehiago
Elhuyarrek garatutako teknologia