Quality and food companies
1996/06/01 Cid, C | Fernández Quintela, A. | Otegi, Itziar | Partearroyo, M.A. Iturria: Elhuyar aldizkaria
On the other hand, the behavior of the current consumer regarding quality has brought as a conclusion that a food company should always try to adapt to the use that is made of its products.

According to Pagola (2), three aspects of customer perceived quality can be defined: the quality of design, product and service.
The quality of the design consists of knowing the will of the customer, making it a measurable precision and subsequently designing a system that guarantees the attainment of the quality required by the customer.
The quality of the product is a consequence of the production process that adapts to the design, that is, the production process without defects.
Finally, the quality of the service is based on compliance with the deadlines and delivery conditions, as well as the willingness and agility of the response to problems after the sale of the product.
When the food company knows what its customer or consumer understands about the quality of its products, it must ensure that it can offer these quality products continuously. And for this it is essential to implement the Quality Assurance System in the company itself, which is defined as a set of pre-established and systematized activities and, as is known, focuses on the products, processes and / or services necessary to ensure compliance with quality requirements. The goal of this system is to ensure the effectiveness of the right product offerings and then to offer confidence.
When making this decision, the implementation of a quality assurance system in a company requires a team of specialized professionals whose responsibility is to guarantee the quality from product design to delivery.
Rubio (3) points out four basic objectives to be achieved by the quality system: avoid errors in any of the company's activities, detect possible defects by imposition of preventive actions, detect the causes that cause them and establish actions to improve products and processes, and objectively demonstrate through different documents that have met the quality requirements.
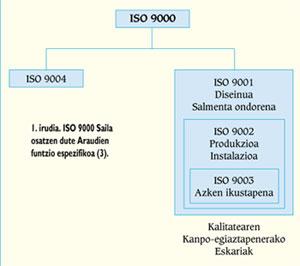
ISO 9000 standards
For the implementation of the Quality Assurance System, companies usually refer to International Standards 9000 published by the International Organization for Standardization (ISO) in March 1987. Subsequently, in December 1987, the European Standardization Commission (CEN) adopted the European Standards of the 29000 Series. The Spanish Organization for Standardization and Certification (AENOR) also incorporated them into the UNE standards of Department 66-900.
The Department ISO 9000, or the Departments EN 29000 and UNE 66-900, are composed of five standards on quality systems whose specific function is included in the scheme of Figure 1. The ISO 9000 Regulation defines the main concepts of quality, that is, the policy of quality, management, certification, control, etc., establishing the general lines of selection and use of the other standards of the same Department. In the event that the company carries out the design of new products, the ISO 9001 Regulation will be incorporated, while in the rest companies will receive the ISO 9002 Regulation.
All these standards define the elements to be taken into account to ensure the achievement of the predetermined quality, setting the requirements related to them (from ISO 9001 to ISO 9003) or offering recommendations (ISO 9004).
In our case, food companies usually use ISO 9002. The standard sets out specifications on the following points:
- Management responsibilities
- Quality system
- Contract review
- Documentation control
- Shopping
- Products sold by customer
- Product identification and traceability
- Process control
- Audit and testing
- Control of inspection equipment and machinery
- Inspection status and testing
- Inadequate product control
- Corrective actions
- Handling, collection, packaging and delivery
- Quality records
- Quality internal audits
- Training and training
- Statistical techniques
In addition, the Quality Assurance System may include other elements contained in ISO 9004 Regulation, such as market research, new product development control, quality cost control, production equipment maintenance, sales control, etc.
As a consequence, it can be said that the quality assurance system is not limited to manufacturing quality, but includes consumers, distributors and other manufacturers and suppliers, affecting the whole company and its environment.
Quality assurance systems
In a food company, quality must have a direct impact on three levels (4):
- Organization quality
- Process quality
- Quality of individuals
Although it can be divided into four areas, ISO 9000 is a model. Consequently, the rest of the elements that will make up the Quality Assurance System of a company must be selected, which is done mainly according to the following points:
- functional and organizational capacity of the company
- complexity of the production process
- characteristics of manufacturing products
- more frequent quality problems
- economic criteria
The implementation of the Quality Assurance System in the company is a difficult task, in which the whole company must participate through different departments.
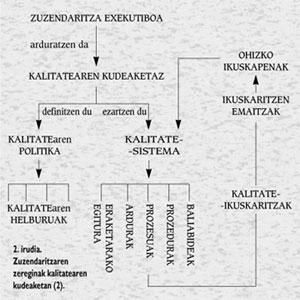
As can be seen in Figure 2, quality management is the responsibility of the Executive Directorate, who must define the quality policy and the general quality objectives to be followed to achieve it. The defined quality policy will be the basis of the quality system to be implemented, that is, the configuration that is activated through a broad audit system when implementing a structure of processes, responsibilities, procedures and resources.
In terms of practice, the implementation of the quality system can be summarized in twelve steps:
- Designate Programme Manager
- Appoint the working committee
- Setting programme objectives
- Distribute quality information within the organization
- Structuring quality and establishing accountability structures
- Assignment of functions to be controlled by procedures (critical activities)
- Define job description
- Development of the Quality Manual
- Consolidate staff participation in the programme
- Preparation of working procedures and instructions
- Set program
- Programme monitoring and review
The first five requirements correspond mainly to the Executive Management of the company. This, in addition to implementing and developing quality policy, will ensure the participation of all members of the organization in achieving the desired quality.
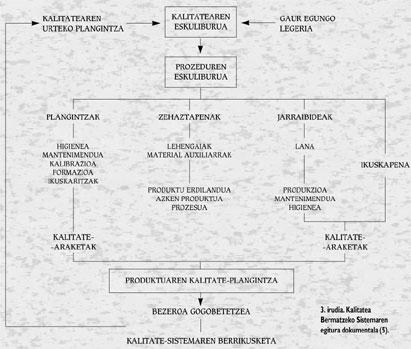
Although the involvement of management is fundamental, the participation of the people involved in the different phases of the process is very important. The most effective way to achieve this is through self-control. According to this method, workers are responsible for the work in quantity and quality. In order to carry out this method it is essential that staff have a good training and an adequate exchange of information between workers and seniors. The advantages that are obtained in this way are important, since it is about reducing the number of workers who will perform the audit or reducing the volume of useless products, among other things because errors are detected immediately. However, for this system to succeed, technical preparation of self-control methods (specifications, measuring equipment, instructions, etc.) should be adequate. ). Figure 3 shows the documentary structure of the Quality Assurance System.
The Quality Manual is a document that describes the quality policy defined by the Management and the structure and quality system of the company for its achievement. It is a unique and constituent document that provides information on everything that is done in the company to achieve quality: control measures, actions, sections and responsible for them. Theoretically it is visible and usable by anyone, but its public character can be reduced at the discretion of the company, and must be approved by the Director General of the company.
The Procedures Manual of the different sections is a structured collection of documents containing mainly technological data. It explains in detail how specific activities are carried out. It includes a detailed description of the procedure, methodology and actions to be used to achieve the objectives set out in the Manual. Unlike the above, it is private and must be authorized by the Quality Committee and, in the case of each procedure, by the corresponding controller.
Other documents are derived from the Procedures Manual: Working instructions, technical standards, specifications, records, etc.
Moreover, the Product Quality Plan is the set of actions to be carried out to ensure the achievement of products with these defined characteristics. It consists of the following operating documents:
- Determination of materials: in terms of raw materials (including auxiliary manufacturing), semi-finished products and final products and throughout the production process.
- Working instructions regarding hygienic conditions of production, maintenance and work. The following points are taken into account in this section:
- Description of operations to be performed
- Quality requirements (product characteristics and defects, sampling planning, specifications, measuring equipment, etc.)
- corrective measures in case of problems.
- raw materials
- machinery
- tooling
- staff training
3. Input, process and final product audit standards.
The results of the aforementioned procedures are collected in the quality records. Includes control graphics and/or roadmaps.
This work of documentation has a great importance, since the statistical treatment of the data allows to know if all the stages of the production are controlled or not, or to detect the errors and their causes, to be able to define later corrective measures and strategies of improvement.
Approval and verification
If you want the Quality Assurance System to be effective, you must offer a continuous improvement basis and organize a continuous process that allows you to achieve total quality, since the sudden implementation of Total Quality is not the most recommended route. Despite what seems curious, the company does not pre-establish the suitability of a quality assurance structure. However, what he asks for is:
- Have a quality policy, objectives and specific settings.
- Keep the quality system documented, orderly and controlled.
In this sense, there are basic differences between homologation and certification systems (5).
The approval is granted by the Administration, is mandatory and is carried out according to the regulations. It is usually set by security issues. Verification is not mandatory and is granted by a private entity. It is established according to technical specifications (almost always according to standards) and its origin is not safety but quality.
The ISO 9000 Regulation defines the Certification as follows: Ensure that a particular product or service meets the requirements set out in a technical standard or specification, using reliable tools created by an entity authorized to do so.
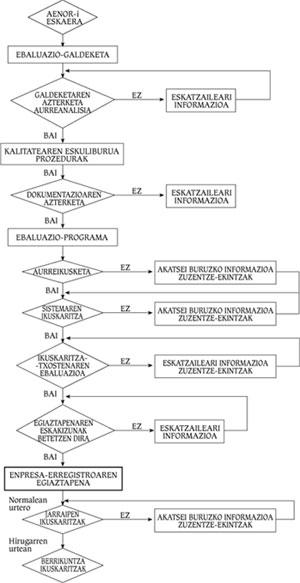
As standards are being implemented, companies have been interested in verifying their quality systems. The verification consists in the analysis of the utility of the Quality Assurance System of the company by a competent organization, but without evaluating or controlling the product. In the Spanish State AENOR is the verification entity and its utility is carried out according to models of Regulations UNE 66-91, 66-902 or 66-903. Figure 4 shows the flowchart of a company's verification process.
Verification process

First, a formal request must be made to AENOR, who will make a questionnaire to the client to know the status of its quality system. A diagnosis will be made after this analysis. If this is negative, the company must correct the errors detected by AENOR. On the contrary, if it is positive, the company will present to AENOR the Quality Manual and the Procedures developed following the model of the ISO 9000 Regulation. Once this documentation has been submitted, AENOR will develop the evaluation programme together with the company. This will set the audit schedule to the system and after this process is completed the report will be presented.
AENOR will decide whether or not the company meets the verification requirements. In the negative case, the company must correct inadequate practices, documents or systems. In case of obtaining it, the company must annually pass the Monitoring Audit to check if the Quality Assurance System that allowed the obtaining of the verification remains in its integrity. During the third year, if no problems have occurred, the Follow-up Audits will be replaced by the Innovation Audits, which should not be overcome so often.
Among the advantages derived from verification are the possibility of using it as a differentiating element in the market, the generation of trust between current and future customers, the exclusion of audits made by customers and the recognition agreements signed by different verification entities.
Although verification is done at the request of customers in other industrial sectors, the first step in the food sector is given by the company itself. In this sense, it risks considering verification as an objective, as a way to demonstrate that the company has the necessary structures, methods and means to guarantee the quality of the products it markets. Therefore, as indicated by Rubio (3), it is advisable that the quality system be tailored to the food company and not according to the verification; subsequently, and at the request of the customers or in view that advantages will be obtained in the market, the system can be adapted to the requirements of the verification. The advantages derived from good quality management are themselves sufficient for the implementation of the quality system to be worthwhile, even if not verifiable.
Finally, we can say that quality is a continuous evolution in the search for improvement in a food company, from quality control to certification or to obtaining Total Quality. To achieve this, the participation of all the people who make up the company and good communication with the consumer are key to keeping the company close to their expectations.

Gai honi buruzko eduki gehiago
Elhuyarrek garatutako teknologia





