Entropy and life
1992/01/01 Mijangos Ugarte, Fernando | Garate Castellano, Kristina Iturria: Elhuyar aldizkaria
The aim of this article is to explain and film the conflict between thermodynamics and life, or if both are not opposed in some points, without proposing, of course, a circular theory. For this, we must recognize that we will rely on classic macroscopic thermodynamics and the chemical evolution of life. We also know that there are other types of thermodynamics (which may be more appropriate to describe irreversible processes such as life, and which are mentioned in this article) and other theories about the genesis of life (such as that of God or that of panspernia), but we will address only those already mentioned.

Our goal is to use as few mathematical and chemical formulas as possible to facilitate their understanding, although occasionally it has been impossible to avoid one or the other.
Classic macroscopic thermodynamics is based on three postulates to which no exceptions have been found. The first two can be easily defined, those that matter to our goal.
The first postulate says that energy cannot be produced or destroyed, even if it can be changed in one way to another. That is, the energy of the Universe is constant. This postulate has direct implications, for example, when all systems move spontaneously to achieve minimal energy, that is, bodies fall from top to bottom and do not rise in themselves.
The water always goes from top to bottom and we have never seen that the water goes from bottom up, or the plane falls off without engine. There are other observations of this type related to this postulate. For example, hot water is placed along with ice and melts, or transistor batteries are exhausted, etc. Somehow, you tend to lower the slope by talking energetically.
Although the above statements are suitable for understanding and internalizing, the first principle of thermodynamics cannot answer the following questions: have you ever seen a large mass of water split into two, one part of hot water and one part of ice? Or that the air of a balloon is concentrated on one end leaving the other part empty? Apparently, the principle of minimal energy in the processes that occur is not enough to explain spontaneity, and thermodynamics poses a new postulate.
The second principle states that: In all the processes that take place in the universe, the disorder increases, that is, the "entropy" increases. This word is of Greek origin and means chaos or disorder. Conceptually it is said that ordered systems have little entropy and that disordered systems have a lot of entropy. Order/disorder is also used as a meter in human sciences. In nature he commands the attitude of disorder. The classrooms are naturally disordered and we have to do an ordination job. Therefore, from this mass of water one cannot properly separate hot water from terror, since this distribution implies the creation of an order.
From a thermodynamic point of view, living beings can be defined as localized regions in which order increases. That is, the appearance and permanence of life on Earth, from structuring to organizational structures that have the capacity to transmit information (understood as a broad definition of life), is a process of ordination that could be a contradiction with the second principle. Therefore, sometimes difficulties or misunderstandings appear.
From now on we will dive into them. It must be remembered that life and its duration are not an isolated process, but an open system, with its environment, with which energy and mass are exchanged. Therefore, the system management process simultaneously involves the process of disorganization of the area and the sum of both is directed towards disorder. It seems paradoxical, but it is so.

We believe that the processes that had to happen 4.6 billion years ago from the constitution of the Earth by condensation of the nebula, to the appearance of the first simplest unicellular organisms of life, 1 billion years later, were very complicated and inert. They probably occurred through an infinite series of small events, and at every moment they occurred it was impossible to retreat, that is, it was an irreversible process. As can be easily deduced, we should use the thermodynamics of irreversible processes, but that is not our goal.
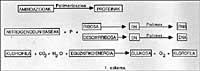
Following the line, we can consider that at this time the Earth was surrounded by reflective atmospheres, without describing in detail its chemical composition. We must say that molecular oxygen (02) was not free, so oxidations could not occur. It is proposed that this atmosphere could be formed by N2, CO, NO, H2O, NH3, always forming a non-oxidizing atmosphere, different from the current one. In this type of writing mosfera, it has been proven that thanks to the ultraviolet light emitted by the Sun (remember that at that time probably there was no ozone) and to all the energy provided by volcanic activity and storms, these inert gases reacted. For example, in 1953, S. Miller showed that in this atmosphere a lot of chemical compounds were obtained, and surprisingly, they are chemical compounds based on life! See figure 1.
To recognize that the passage from the inorganic to the organic can be very simple and to know that it is something eternal (something like the claim that the Earth is round), we know from an experiment conducted by Wöhler in 1832 (it showed that an inorganic compound, ammonium cyanate, could obtain a heated organic compound, urea). It has always been considered that the organic framework was related to an unknown motor force, called "life force". Today we know that the passage from one field to another is absolutely chemical.
Miller's sessions showed that many of the fallen organic compounds in the atmosphere were packed in the water (see Figure 1 and Trial Description). The passage of these inorganic raw materials to these organic compounds implies the passage from disorder to order, all due to solar energy. It is somehow used to order energy.
Polymerization
Explaining why these monomer molecules joined together to become complex organic chemical structures (which have the ability to store chemical information), it is much more difficult to explain why the process known as polymerization occurred, and we will make some considerations to clarify them.
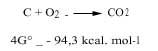
Chemists study two aspects of thermodynamics, that is, the postulate of minimal energy and entropy, to know whether a chemical reaction can occur naturally or not. To combine these two positions we use the energy change of Gibbs: if at a certain temperature the energy of Gibbs is decreasing, we say that this reaction can occur spontaneously. For example, coal is burned along with oxygen, as the energy variation of its Gibbs is negative, as can be seen in the following reaction (hence the oxidizing atmosphere in which oxygen is free is oxidizing):
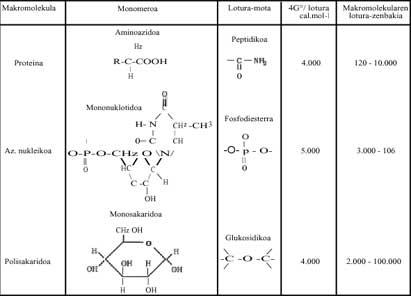
In polymerization processes, when simple molecules (monomers) must form sugars, lipids, proteins, nucleic acids, etc., Gibbs energy is increasing, so it does not seem that the reaction can occur spontaneously (see Gibbs modified energy modification of some of the polymers written in Table 1 below). What happens? In living beings, in our bodies, we have responded by saying that these reactions are always happening (otherwise we would be dead), but how and why do they occur?
It must be said that intracellular reactions (considered for simple living elements) do not occur in isolated systems, but that the cell itself is an open system, that is, there is an exchange. Polymerization reactions are due to the simultaneous production of other highly exergonic reactions (from which Gibbs release energy).
These exergonic reactions are those that release a lot of energy. Metaphorically speaking, they are local chemical solar. This released energy helps polymerization. Without Bes, we are talking about Adenosin Triphosphate (ATP), which by hydrolyzing releases a lot of free energy. All polymerizations that occur in living beings are due to ATP (See Table 1). It can be written briefly:

The ATP molecule is called energy storage. Some polymerizations may occur without ATP, probably because equivalent molecules like it intervene.
If the current polymerizations occur thanks to ATP, being a totally universal mechanism, how could they be produced in the aforementioned time? Was ATP already formed in this chemical evolution of life? These questions, of course, have nothing to do with thermodynamics and we chemists cannot answer them correctly. However, this Table 2 has written several molecules that could be used as a "local sun" and can be easily synthesized in Miller's trials. Chance? What must be made clear is that the polymerization processes, that is, the passage from disorder to order, could occur with the help of reactions that release free energy (or Gibbs energy).
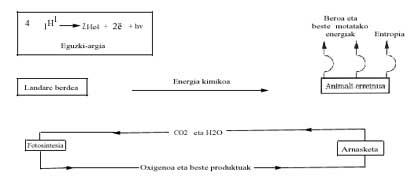
From the energy point of view, the energy balance of current life and its possible consequences are analyzed below. We know that the Sun emits a lot of energy and makes a long journey and makes "filters" in the Earth's atmosphere (ozone layer, etc.) Once overcome, it reaches the terrestrial surface. Visible energy is used only in the process called photosynthesis, where glucose (C6H1206) from CO2 and H2O raw materials is synthesized. Although Gibbs' energy change is endergonic in this reaction, the energy supplied by the Sun allows its realization, according to the following equation:

Green plants use this glucose at night and burn it with oxygen in order to carry out other polymerization processes with the energy released. The animal kingdom takes advantage (eats) of the chemical work of the photosynthesis of green plants and gets energy thanks to the combustion of glucose (or fat or other substances).
If this burning occurs as burning wood or coal in the fire, it could immediately be deduced that only the "body" would be burned, that is, that the energy released in the burning would break the chemical bonds of the biochemical compounds that would be degraded by the administration of C02 and H2O.
The evolution of life, Nature, faster than here exposed, has undergone a natural evolution through a mechanism of progressive release of this energy, which also accumulates it as chemical energy. This ATP molecule comes to mind again.
Living beings consume energy to carry out different types of work, that is, processes that would not occur by themselves as defined by classic macroscopic thermodynamics. These processes can be expressed as:
- Trace elements and raw materials, i.e. “food”, must be transported, transported and transported to all the cells that make up the living entity. To do this you have to work and this concentration and / or transport is not easy. Have you ever wondered what tiredness it is necessary to bring up the water and the salts from the roots to the top of a tree? or how is the pumping the heart has to do to send blood to the finger and the energy it consumes? Many things must be transported and concentrated in the bodies.
- In addition, much energy is needed to polymerize, for example, to synthesize DNA molecules that have the ability to transmit genetic information from their ancestors to their descendants. That is, to ensure the survival of life, energy is needed. (In short, we are still alive because we feed in the short term, but only if we reproduce as a species in the long term).
- Finally, we do some physical work such as muscle contraction… and any work that gives off heat. That is, the heat that gives off the living being, the one that absorbs
the medium and therefore increases the local entropy. Therefore, in this natural, living process, the entropy or disorder of the universe is increasing.
According to the above, it can be stated that: That the appearance and development of life on planet Earth are not processes contrary to the second principle of thermodynamics. We wanted to explain one of them that fulfills the principle. If we say that the truth is round, we should recognize that we have only made an approximation, and in the following lines we will try to make it manifest.
On the one hand, the classic macroscopic thermodynamics used predicts changes that can occur in balanced macroscopic systems and thus has been applied to the cell. However, reflecting we will realize the weakness of this approach. We know that the cell is very microscopic. Therefore, macroscopic concentrations and concentrations in them can be very different. Moreover, the cell is not an isolated, but open system, as mentioned above, so it is an unbalanced and unbalanced system. However, the concentration of large quantities of raw materials remains virtually constant over time. That is why this situation is called a stationary state (false balanced).
The appearance of life has basically been a natural and therefore irreversible process. In this case it would be much more appropriate to use irreversible thermodynamics than classical thermodynamics, whose description is outside this article.
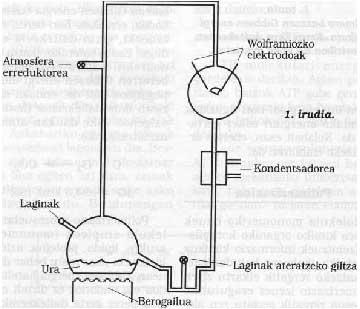
At the University of Chicago S. Miller and H. Tooling scheme used by Urey. Their experimental conditions were simulated in the primitive Earth. From the key on the left was introduced an atmosphere of drafting gases, which was excited with a strong electric spark (at that time can be considered energy emitted by the Sun). The water of the lower flask was heated (at that time the Earth was considered to be very hot) and cooled by a condenser falling in the form of raindrop. The compounds dissolved in these drops of water accumulated at the bottom and with the key of it samples were periodically extracted.
15% of all methane carbon introduced in these sessions had become a biochemical compound, such as glycine, alanine, glutamic acid, urea, etc. (at least twenty different compounds) and another large percentage in the form of tar sand. It is surprising that most of the amino acids that form proteins can be obtained in these sessions and even more so in most life-sustaining compounds. Is it pure chance or could life appear with the evolution of these compounds?


Gai honi buruzko eduki gehiago
Elhuyarrek garatutako teknologia