Ozone: towards consensus
1990/01/01 Aizpurua Sarasola, Joxerra Iturria: Elhuyar aldizkaria

Next May marks five years Joe Farman, B. Gardiner and J. Since the Sres. Shanklin published his research in the prestigious journal “Nature”. We “Elhuyar. In issue 8 of “Science and Technology”, that is, in April 1987, we first published ozone news. Since then and until three years have passed, the ozone problem has been a rich news source that can be a good occasion to explain the evolution of this mental disorder. This explanation begins in the aforementioned article.
If we look at the title of that article, entitled “Decreases the ozone level”, we can say that it is currently fully valid, since since then the “hole” has increased.
Waiting for what current research says, there are four big hypotheses that try to explain this phenomenon, things have changed a lot. These investigations allowed us to overcome a hypothesis about others and even confirm. Therefore, today no one puts the focus of the problem on emissions into the atmosphere of our society. Also known is the type of substance that most contributes to the impact of the ozone layer, that is, the chlorofluoro hydrocarbon family (CFC). This does not mean that there are no other harmful substances, but their ozone disease rate is lower. For example, nitrogen oxides emitted from fossil fuels damage ozone, but not the size of CFCs.
In this diagnosis most scientists and researchers agree, although the entire structure of the phenomenon has not yet been understood.
But when and where does the ozone hole occur?

It is well known that so far the alarm has occurred in the South Pole. Therefore, this will be the geographic location of our study.
Through natural phenomena that we will not study in these pages, the winds in the stratosphere tend to go from the equator to the poles. Therefore, substances in the stratosphere, whether polluting or not, are also directed towards the poles. Likewise, the polar atmosphere is rarely renewed, so the polar atmosphere is considered a special chemical laboratory. Scientists have realized this situation, so for some time research has begun in these places. Specifically, ozone levels have been measured since 1957.
The unit most used and used to measure the amount of ozone is the dobson unit. It is defined as:
“If the ozone molecules in a vertical cylinder with a foot of 1 cm 2 are put into a pressurized atmosphere and at a temperature of 20ºC, a dobson unit represents an ozone layer of 0.01 mm.”
If we measure ozone levels at different points on Earth, we will realize that it is completely variable, both geographically and over time. The ozone level can range from 200 to 500 dobson, i.e. from 2 to 5 mm.
Returning to the South Pole, it was observed that measurements made until 1980 changed ozone levels between 280 and 350 doson, reaching a minimum in a contracted period each year. This period occurred in September or October, when the change occurs from winter to spring.

Measurements made since 1980 generated gravity among scientists. Ozone levels decreased by 20% year after year. The minimum to date occurred in 1987, approximately 110 dobsons. In 1989 the minimum exceeded a similar value. We want to reiterate that these lows occur in the last weeks of September and in the first weeks of October. So, reader, right now there are no holes in the South Pole.
The origin of the problem was determined in 1988. Although previously suspected, until this year the chain of reaction that occurred in the atmosphere was not clarified.
The reaction procedure in the South Pole stratosphere, without entering into precise chemical terminologies, is as follows:
“When chlorine nitrate (ClONO 2) present in the atmosphere is associated with hydrochloric acid (HCl), nitric acid (HNO 3) and the chlorine molecule (Cl 2) and hypochlorous acid (BSl) are formed. When the sun appears, both the chlorine molecule and hypochlorous acid dissociate into Cl.”
The Cl atom attacks ozone according to the following formula:

Hypochlorous acid is then dissolved by the action of the solar rays and the released Cl atom retakes the reaction (1).
An average chlorine atom can destroy 100,000 ozone molecules.
The few reactions mentioned require very special weather conditions. On the one hand, in the stratosphere a temperature of less than -80ºC is needed and, on the other, solar rays are needed. Finding these two conditions at once is very difficult. This only happens in the past winter to spring. When the sun sounds, it warms the stratosphere and makes it impossible to carry out the reactions described above.
Although the chlorine atom has been found to be the main culprit, in recent months the presence of another more harmful substance, bromine, has been detected. However, since the amount of chlorine present in the atmosphere is much higher than that of bromine, we will place chlorine in the first position on the list of ozone aggressors.
Once the responsibility for the halogenated elements (Cl and B) was verified, it was easy to determine the harmful substances. Substances emitted into the atmosphere from our industries containing halogenated elements are substances of the CFC family. These substances are called freons (with Cl) and halons (with Br) in street language.
The following scheme shows the use of CFCs in Europe:
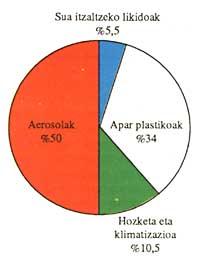
CFCs production has grown year after year. The most commonly used substances are CFC 11 and CFC 12, but they are not the only ones that can be observed in the following scheme:
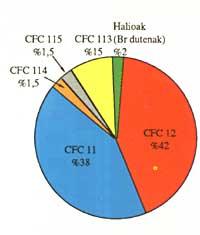
The global production of CFCs estimated at this time is 1.15 million tons and the median of each substance can also be seen in the following table:
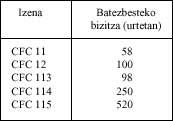
These substances do not rise rapidly into the stratosphere when they are emitted into the atmosphere. The rise is very slow, that is, it is estimated that it takes between 10 and 20 years. According to this, the substances that now attack ozone are those emitted in the 1960s to the 1970s. Given that CFCs production has grown dramatically from 1970 to the present, a very serious future can be predicted. century. It is a good time to say that nature is nothing linear, that is, although the data provided predict a black future, nature has defense mechanisms that we do not know well and, therefore, when making statements about the future must have great uncertainties.
So far the problem has emerged especially in the South Pole, but in recent years the first alarms have occurred in the North Pole. In addition, given that most of the world's production takes place in the northern hemisphere, there seems to be cause for concern. But why has there not been such a serious situation in the North Pole as in the South Pole?

To answer this question it is necessary to analyze the climatic conditions of both places. The South Pole is surrounded by water, so this water keeps its atmosphere practically isolated. On the contrary, being the North Pole surrounded by continents, air change is more frequent. However, the appearance of the first symptoms has caused industrialized states to be in a state of relative gravity, very close to the North Pole.
When ozone is in the stratosphere, it has very good effects for life, as they do not let ultraviolet rays pass. But when it comes to pollution in the streets of our villages, it can be very harmful to our health. The presence of the most industrialized States in the Northern Hemisphere means that the amount of ozone from the pollution pathway is not negligible. This ozone accumulates in the troposphere (that is, between 10 km of soil height) and a few months ago it has been discovered that this ozone also prevents ultraviolet rays. Good luck! This is nothing more than a surprise that nature can offer.
The title of this article used the phrase “in agreement”, since there is a consensus issue. The greatest discrepancies still occur in the resolution of this problem. It is clear that if the production of harmful substances was completely cut off, the ozone problem would be in the process of solution, but these are only pretty words. How many unemployed people? How to replace some essential products in our society? how to guide the economic planning of some developing countries?
Some of the questions that define or condition the solution are:
At an international meeting held in Montreal in 1987, the first decision was made to solve the problem. It approved:

“2000. By 2004, global production of CFCs should be halved by 1986.”
But that same year the low levels of ozone reached in the South Pole showed that that decision was not enough. Subsequently, the international meetings in Helsinki and London have made more severe decisions, leaving the approval of these decisions to each nation.
In general, advanced or developed nations are more likely to disrupt or replace production than developing nations. Replacement products, if available on the market. Some of them belong to the family of CFC substances and although they do not damage ozone, not knowing their properties well they look with great distrust.
“… the decrease in ozone would increase the amount of ultraviolet rays emitted on Earth and, therefore, in addition to the skin cancer mentioned above, the death of plants and animals, mutations, alteration of the atmosphere, etc. So we finished our article. This statement remains valid today.
Laboratory tests, at least on a small scale, have demonstrated in practice the theoretical effect of increasing the level of ultraviolet rays. In addition, CFC substances, like carbon oxides, contribute to the greenhouse effect. Therefore, the reader should start making data decisions.

Gai honi buruzko eduki gehiago
Elhuyarrek garatutako teknologia





