Rare Earth
1990/11/01 Barrenetxea, Tere - Elhuyar Fundazioa Iturria: Elhuyar aldizkaria
The 17 metals between atomic number 21 and 71 chemical elements and the compounds that produce them are called rare earths.
These seventeen elements are: Three elements of group III b [Escandio (Sc), Itrio (Y) and Lantano (La)] and the first of the two series, commonly called lantanide, are written outside the periodic table, composed of 14 elements. The name of lantanide is due to its offspring. The lanthanide series consists of: Cerio (Ce), Praseodymium (Pr), Neodymium (Nd), Prometio (Pm), Samario (Sm), Europio (Eu), Gadolinium (Gd), Terbio (Tb), Disprosion (Dy), Holmio (Ho), Blutitorio (Blu).
History
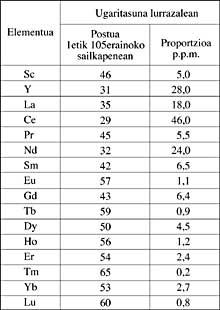
Rare earth name, XVIII. Since the 20th century, when the first discoveries about these metals were made. The proportion of these metals was minimal in those minerals from initial studies. Given this scarcity, these researchers believed they were very rare on the earth's surface. Later it has been proven that they were wrong. Cerium, lanthanum and neodymium are more abundant on the earth's surface than lead. Yttrium is more common than tin and the earth's crust is as rich in tulia as in iodes.
The scientific history of these elements is very turbulent. The investigation of many years of hardness, mixing and step by step after the accumulated knowledge until today. Between the first discovery of an element of this family and the last to isolate spent 144 years. It took half a century to discover that didymium was a mixture of neodymium and praseodymium.
The long history of rare earth research was born in 1794. That year the Finnish chemist Johann Gadolin isolated the first rust from the rare earth family. It isolated the rust of a rare earth from a mineral obtained in the Swedish municipality of Ytterby. He believed he had a new element he baptized as an iterbit. Finally, in short, it was called itria.
Years later, in 1803, researchers such as Martin Heinrich Klaproth or the famous Jöns Jacob Berzelius isolated another rare land, also rust. It was given the name Zeria in honor of the newly discovered asteroid Zere. This was also a pure element. In 1808 Sir Humphry Davy demonstrated that the rare earths discovered until then were not elements, but combinations between metallic elements and oxygen. Metals that provided rust and itria were called cerium and itrium.
Over the course of forty years it was considered that the itria and the artery, that is, those that were considered oxides of yttrium and cerium, were chemically well defined. But they were really mixtures of rare earth oxides. In 1840 the Swedish mineralogist Carl Gustav Mosander finally achieved the division of the Itria and the Barber in rare lands. This was the discovery that opened the door to the isolation of the entire rare earth succession.
In the following years, numerous investigations were carried out, but it was difficult to advance. Great confusion was created around certain discoveries, and in order to clarify whether these were pure elements or confusions, numerous publications were made. The names of the elements also changed from laboratory to laboratory.
In 1869, when the Russian chemist Dimitri Ivanovitx Mendeleiev proposed the periodic table, he foresaw the need to leave a gap for the scandal. In 1871 it also foresees some features of this element. When the Eskhandium was discovered in 1879 and its characteristics were reported, it was observed that they conformed to what was foreseen by MendeleiEV. This caused the Mendeleev table to be accepted among scientists.
The investigations continued with hardness, which allowed to isolate the lutetium in 1907, the last element of the series.
But the truth is that the element promised was not isolated until much later. When Henry Moseley assigned to each element of the Periodic Table an atomic number, it was possible to know exactly where the free holes were. Until the years 1913-14 and Moseley discovered that lanthanids could only be 14 (from heaven to lutetia) and that atomic number 61 was the only one yet to be discovered. However, it was not isolated until 1947. He was called Prometio.
Why has it been so difficult to identify and isolate these elements? The reason may be that these elements are found in the same mineral and have very similar chemical characteristics. That is why they have been hiding for so long. The most lethargic is the group of elements with more similar characteristics. Analyzing a metal given by pure in a discovery with the new techniques developed in the coming years, on more than one occasion it could be verified that it was a mixture of two or three rare earths.
An advantage of having such similar chemical properties is that, in industrial use, when these properties should be used, it is not worth a very fine distribution. This distribution is necessary when the characteristics to be used are physical characteristics.
Electronic structure
Next we will see how the electrons of these elements are arranged. Electrons are known to be layered around the nucleus of the atom. A quantum number n and for lanthanids n = 6 corresponds to each layer. Each layer has a certain number of electrons and these are organized in turn into sublayers. These sublayers are "s", "p", "d" and "f".
As the atomic number increases, the number of electrons also increases. The electronic structure of the previous element will be added a new electron by which it is related below. The added electron will not be located anywhere. A series consists of the set of elements that correspond to the number of electrons needed to complete a sublayer in formation.
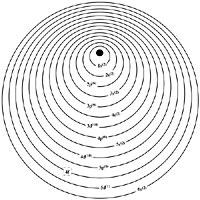
The most stable electrons are the electrons "s" and "p", so they tend to be placed in these first sublayers. At the end of the fifth period of the Periodic Table, sublayers 4s, 4p, 4d and 5s and 5p are complete. The sixth period begins with the incorporation of two 6s electrons and the third goes to orbital 5d forming the element called lanthanum. The fourth electron is directed to sublayer 4f, until then empty. Since this sublayer has capacity for 14 electrons, the series is composed of 14 lanthanids, from zero to lutetium.
The yttrium and the scandium that make up the rare earth family are found in the same column of the periodic table in which the lanthanum is found. The most superficial scanning electrons are two 4s electrons and a 3d electron. In the case of the yttrium, the electrons 5s and 4d.
First rare earth uses
The cerium was the first to gain fame and fame among the rare earths and to some extent to provoke research into the whole family.
The discovery made by Baron Auer von Welsbach in 1883 is at the basis of the interest aroused by heaven. The baron published a new lighting method that year. Welsbach wet a tissue in dissolution of thorium salts, cerium and other rare earths. He then discovered that the fabric gives bright light by putting it in heat from a gas flame. It was a very surprising discovery for this time. This is a feature that is still used today, as it is the mode of operation of the refractory shirt of the camping gas lamps.
Years later, in 1903, Auer von Welsbach himself discovered another use of rare earths: the lighter stone. Cerium is a pyrophide, that is, it has the facility of spontaneously inflating in the air and of removing sparks rubbing. The cerium had to be alloyed with iron to be used in lighter stones, so that it did not feed on the fire. This is the first step to using rare earth in metallurgy.
A common feature of these two uses is that they do not require elements of great purity. Therefore, they could be exploited industrially in those times when chemical distribution methods for these uses were not so developed.
Current industrial uses
Metallurgy

Between 1960 and 1974 an alloy called mischmetal, composed of lanthanes, pigs and neodymium, experienced a spectacular boom in the processes of casting and purification of steel. Due to the affinity of this alloy with oxygen and sulfur, it is suitable for use as deoxidizing and desulfurizing. Improves anti-corrosion properties.
In cast iron, the materials are more resistant, ductile and therefore easier to process.
Currently the use of this mischmetal is reduced by increasing the use of calcium compounds in desulfurization.
Catalyzed reactions
Rare earths are widespread in chemical reactions such as polymerization or hydrogenation, which serve to accelerate or modify. Lanthanides rarely act as authentic catalysts, their role being to ensure the stability, activation and selectivity of the catalytic system.
In oil cracking, for example, crystalline structures called zeolites are used as catalysts. They consist of silicon and aluminum and alkaline elements such as potassium and sodium. Replacing sodium with a rare earth mixture improves the catalytic characteristics and effective life of zeolite.
Catalytic vehicle boat
Catalysts are used in the catalytic canister to stabilize, optimizing its operation and extending its life. Catalytic pots are obtained by applying active components (platinum, palladium and rhodium) in a structure similar to the bee cells formed by aluminum and cerium or lanthanum oxides. In countries with the most demanding anti-pollution legislation, the use of the catalytic boat is absolutely necessary, meaning hundreds of tons of rare earths are needed annually.
Glass and ceramics
The glass and ceramic industry is one of the largest rare earth consumers. Rare earths selectively absorb and emit several wavelengths of the visible and ultraviolet spectrum. Therefore, the colors obtained are very clear, very defined and stable. Therefore, rare earths are used as dyes and even as luminophores.
Although it may seem otherwise, rare earths are also used to obtain colorless glass. Adding to a red glass a strange earth that selectively absorbs red, you get a colorless glass that rebalances the color spectrum.
Glasses with rare earths withstand sunlight very well, so they are used not only for windows but also for sunglasses.
Color TV screen
As mentioned above, the first industrial use of cerium oxide was the Welsbach lighter, as Auer says, or others. We know that what is below this use is a characteristic that becomes luminescent with heat. This result was extremely surprising in those times, so there was great interest among scientists and non-scientists on rare earth. But it has been necessary to wait until the 1960s to be able to value this physical property.
By then, let's keep in mind that by the 1960s the methods of chemical distribution were much more developed and a colorful television appeared. The luminophores, that is, the sensitive aspects of the cathodic tube, besides having a good luminosity and a long duration, must give a very well defined color. Since the Europe-activated Yttrium vanadate meets these requirements, it is used on color TV screens.
These are the characteristics that justify the use of the europium in fluorescent lighting.
Permanent magnets
In 1969 the researchers discovered that with the introduction of samarium in a cobalt alloy an extremely powerful durable magnet was obtained. This magnet is very resistant to disharmony and is 30 times stronger than traditional magnets and six times stronger than ferrite (the best hour magnets).
With rare earths you can get much smaller magnets that have the same strength and so you can get to the ditxoso Walkman, so well known today. The advantages offered by samarium and cobalt magnets are so high that they are used in most low and medium power motor electromagnets, although their price is very high.
In order to overcome the price barrier, more research has been done and in 1983 it has been detected that magnets composed of ferboros and neodymium have the right properties. This way you can avoid using a metal as expensive as samarium. This new material does not maintain its characteristics above 300ºC (while samario-cobalt keeps them until 700ºC), which makes it difficult to use for certain functions such as vehicle starter engines.
By superconductivity

Superconductivity consists in the disappearance of electrical resistance and therefore of energy dissipation. Many materials present this property near the absolute zero (-273ºC).
Studies of recent years have found materials that present this property at higher temperatures. In 1986, a lanthanum, barium and copper oxide was found as a superconductor at -243ºC. A. Müller and J.G. German physicists Bednorz. For this work they received the Nobel Prize and opened a new stage in the field of superconductivity at higher temperatures.
The new studies carried the limit up to -183ºC with ternary oxides with rare earths. Today the record has reached -148ºC and nobody knows how far researchers will be able to carry this limitation in the coming years.
The above uses do not close the list. Radiology, lasers, adjustment optics and jewelry are fields that take advantage of rare earth properties.

Gai honi buruzko eduki gehiago
Elhuyarrek garatutako teknologia