Conductive rubber
1989/01/01 Elhuyar Zientzia Iturria: Elhuyar aldizkaria
Over the past decade, chemists have developed sophisticated methods to convert polymers into conductors. According to Thakur's work, the most common polymer, rubber, has been discarded.
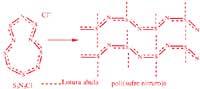
According to chemists, in order for the polymer to be conductive, the atoms of the polymer chain must be joined by a double conjugated bond (that is, by alternative single and double bonds). This facilitates the movement of electrons through the chain. When an electron moves it leaves a positive hole. This hole will fill another electron, which will leave a new positive hole. Conductive polymers drive because electricity, holes and electrons move from one end to the other of the chain. Polyethylene is one of these.
Most polymers, such as rubber, have no double conjugate bond. Rubber consists of units consisting of 5 carbon atoms that are repeated several times and in each unit there is a single double bond.
Iodine is a chemical oxidant that attacks the double bond. In addition, iodine makes rubber a conductor. Iodine extracts the electrons from the double isolated bonds leaving the holes. The holes jump from the chain to the chain and electricity can fly as in the case of conjugated connections.
Other polymers such as rubber have important advantages over conventional conductive polymers. Having fewer double joints, they are more flexible and easier to mold and handle.

Gai honi buruzko eduki gehiago
Elhuyarrek garatutako teknologia