Carbon, the basis of life
2003/01/29 Roa Zubia, Guillermo - Elhuyar Zientzia

Life needs molecules of different sizes and types. Some small, about twenty atoms, others larger and others giant, with thousands of atoms. Some are for storing energy and others for spending. Some are mixed with water and others are similar to oil.
Each of them has a specific function that requires a concrete structure. DNA, proteins, vitamins, sugars, lipids… all are carbon-based.
But the question we are going to ask the other way around, why don't they have other chemical elements as a living value base?
In nature there are 92 elements, with hydrogen being the lightest and uranium the heaviest. All of them, except one, have several reasons not to be the basis of life. Let us consider these reasons:
Five of the 92 elements are radioactive, so over time they disintegrate: astato, radón, francia, actinio and prometio. It is clear that the atoms that disintegrate cannot participate in life.
Five other noble gases are helium, neon, argon, crypton and xenon. They do not constitute a chemical relationship with other elements, either with each other or with oneself. But life needs complex and stable structures to move forward, that is, atoms that unite and do not break down are essential.

The 64 metals present in nature are also not suitable for creating life, with an insufficient number of electrons. To cope with this scarcity they need a very special chemical bond: many atoms are joined together and electrons are distributed among all.
But for this system to be effective it is necessary to unite thousands of atoms. That is, there are no structures formed by ten or twenty atoms of iron, copper or silver. In life, however, these small structures are essential.
Only 18 elements remain in the list of candidates for life. These elements do make the proper chemical bonds and do not disintegrate. But seven of them tend to unite in pairs: hydrogen, fluorine, chlorine, bromine, iodine, oxygen and nitrogen. They form structures of two atoms.
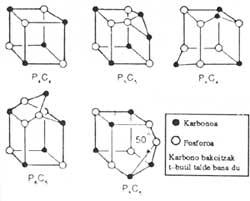
Three others, sulfur, selenium and tellurium, join together forming chains and four others, boron, phosphorus, arsenic and antimony, join together in flat structures.
On the contrary, life needs three-dimensional structures that only four elements remain: carbon, silicon, germanium and tin.
At least they are four and not one. So why is carbon the only one in living beings?
Germanium and tin form very weak bonds. They are not structures of great stability. Silicon, for its part, forms much stronger relationships with other elements than with itself. Most of the silicon in the soil is related to oxygen, which forms the stones called silicates.
Therefore a life based on silicon is not possible, because the molecules of life would oxidize quickly.
Therefore, the only option is carbon.
Of 92 elements, only one.
Perhaps we should thank nature for having at least carbon.
Text prepared for the radio program Norteko Ferrokarrilla.

Gai honi buruzko eduki gehiago
Elhuyarrek garatutako teknologia