Ice energy: gas hydrates
1988/12/01 Barandiaran, Mariaje | Irazabalbeitia, Inaki - kimikaria eta zientzia-dibulgatzaileaElhuyar Fundazioa Iturria: Elhuyar aldizkaria
Much of the terrestrial surface is occupied by gaseous hydrocarbons trapped in the ice. The gas molecules are stored in ice formed boxes and it is estimated that the amount of natural gas so stored can be a hundred times greater than that in the form of free gas. Although the exploitation of these gas hydrates poses technical problems, it can serve to partially meet the energy needs of our civilization.
According to some researchers, for example, the Soviet Juri Makogon could explain the formation of planets of the solar system by gas hydrates. On the other hand, Comet Halley is formed by hydrates of gas.
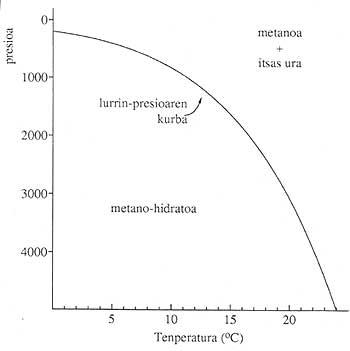
Gas hydrates are found in many parts of the terrestrial surface (continents and oceans), provided the pressure and pressure conditions are adequate. It requires low temperatures and high pressures. 1. The figure shows the stability conditions of the gas hydrates that form in the oceans. Almost all light gases (methane, ethane, carbon oxides (II) form solid compounds with water molecules and in suitable conditions.
A little history
Gas hydrates are not new compounds, since in 1810 the well-known English chemist Humphry Davy accidentally obtained a chlorine hydrate. In his laboratory, at a temperature of 9ºC, he obtained chlorine hydrochloride flakes mixed with chlorinated water. It was not then that he took heed.
For years, gas hydrates were forgotten and marginalized. However, in the 1930s the interest reemerged in its surroundings, when it was discovered that the obstructions of the pipelines of the polar regions were due to the hydrates of gas. To avoid obstruction of the pipelines, it was necessary to dehydrate the gas.
However, the boom in gas hydrates dates back to the 1960s, when it was observed that gas hydrates are found on the terrestrial surface and on the soil of the oceans. Then they began to see the gas hydrates as a source of energy.
Characteristics of the gas hydrates
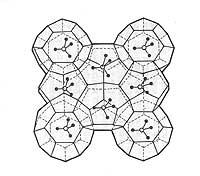
According to chemicals, gas hydrates have a basic three-dimensional structure. This structure forms holes inside as a box, where the gas is trapped. Gas hydrates are familiar known as clatrates among chemicals. These compounds are solid and have form of ice or spongy snow.
The gases of molecular size less than 0.69 nm produce easily hydrates of gases. But larger gases, such as butane and ethane, need smaller gas molecules (such as methane) to form a hydrate.
In the hydrates of gas two types of structures are known. In the structure called I, the gas is trapped in 46 molecules of ice and only supports gases of small molecular diameter. The other structure, called II, supports large molecular diameter gases and is made up of 136 water molecules.
The gas hydrates that form in the pipelines have a generally confused structure. A very interesting feature of the gas hydrates is the speed of propagation of the seismic waves themselves. In porous saturated means of gas hydrates, the speed of propagation of the seismic waves is between 60 and 100% higher than that of the saturated zones of free gas. This makes it possible to differentiate ice, gas hydrates and free gas, a method used to find gas hydrates in the sediment layers.
Another interesting feature of the hydrates is its impermeability. Gas hydrates are very waterproof to gas and water, more waterproof than water-saturated clays that work as a cover for gas and oil sinks. This self-obstruction allows the formation of large deposits of gas hydrate in the oceanic soil.
Methane is the most abundant gas in gas hydrates, although it can also be found ethane, propane, butane, hydrogen sulfide and inert gases.
Formation of gas hydrates
There must be adequate conditions of pressure and temperature to form gas hydrates. The best regions on earth to form gas hydrates cover 25% of the continents and 90% of the oceans. Therefore, and taking into account that 3/4 of the Earth are covered by oceans, there are huge reserves of gas hydrate.
The natural gases are free in the holes and cracks of the sedimentary rocks, or dissolved in the water, but when the appropriate conditions are given they become solid and precipitate as hydrates of gases. Isotope studies carried out in methane hydrates present in the seabed graves have their origin in the decomposition of organic matter.
For this formation to occur in the appropriate regions for the formation of hydrates, it is necessary to ensure a quantity of gas that keeps saturation in the water. When the hydrate precipitates on the pore of the sediments, the concentration of gases in the surrounding water decreases considerably. This decrease in concentration attracts the gas that is found in the surrounding stones to achieve balance and this gas attracted precipitates increasing the concentration of hydrate. It is because the gas hydrates are waterproof to gas.
Exploitation of gas hydrates

If the hydrates of gas are proposed as a source of energy, the methods of use of the sinks of the same should be studied. Gas hydrochloride sinks can be found in two very different areas (on land and sea), so the operating routes must be different.
On the ground, there is a general axis that consists of releasing gas from the gas hydrate for later use by conventional methods. The phase change of the gas can be done by different ways.
If the pressure decreases in the pit, the hydrates of gas begin to decompose. Thermodynamic and electroacoustic methods can also be used. The decomposition of the hydrates can be obtained by injecting hot water or steam. Although it is not known exactly, the energy needed to release the gas trapped in hydrates is not much greater than the one needed to melt the ice. Some believe that part of the methane released by the pit can be burned to heat the pit.
The submarine deposits have special characteristics, since they are not covered by a layer of impermeable rock. In addition, they are very widespread in the seabed and their depth can vary between one and one hundred meters. But being subjected to large amounts of water, its constant hydrostatic pressure exploitation is possible regardless of the decomposition route of the hydrate. Another factor to take into account in the underwater graves is that they usually constitute a waterproof cover of free gas tanks or petriolium. Therefore, in this case it will be necessary to exploit free gas or oil before the gas hydrate.
The hydrates of gas can be a source of energy for the future, but it is still not enough with those known to exploit the hydrates of gas. Throughout the world, and especially in the Soviet Union, this aspect is being investigated.

Gai honi buruzko eduki gehiago
Elhuyarrek garatutako teknologia




