Ebola vaccine available for 2015
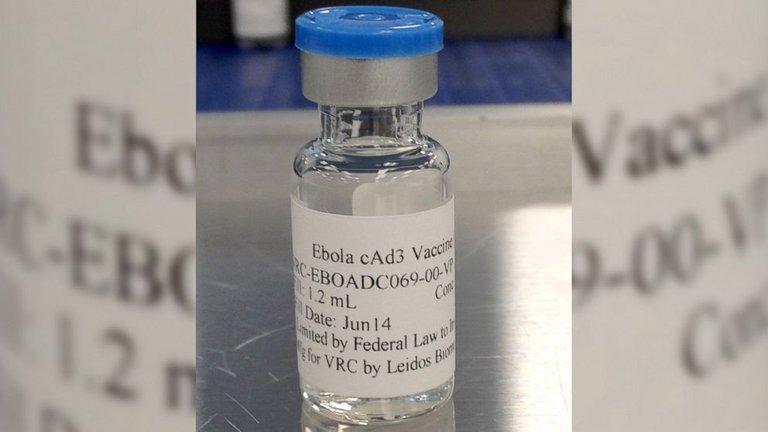
Ebola is a phylovirus with seven genes. It is introduced into the human organism by mucous membranes, initially affecting monocytes, macrophages, dendritic cells and liver Kupffer cells. In turn, it inhibits immune system protection mechanisms. Therefore, to be effective, vaccines must be able to address this double strategy.
One of the two most advanced vaccines is a chimpanzee adenovirus with a superficial Ebola protein. The pharmaceutical company GlaxoSmith Kline and the U.S. Department of Infectious Diseases are being developed and tested in the United States, Britain and Mali.
The other is a recombinant vesicular stomatitis virus developed by the Canadian Health Agency and produced by the company New Links. They are testing in the United States and hope to try it soon in Europe and Africa.
Both are in the first phase of clinical trials. At this stage, they must demonstrate that vaccines are safe and generate immune response. In addition, they should measure the doses needed to generate this response, which allows them to know when vaccines may be prepared.
Obstacles
However, before starting vaccinations, they must pass the other phases of clinical trials. The second and third phases aim to ensure efficacy and safety, and the two vaccines mentioned are scheduled for December and January in Liberia and Sierra Leone. To do this, they need a large number of volunteers: each vaccine has to be tested by 10,000 people and so many others must receive pleasure. A placebo free trial will also be conducted in Sierra Leone to speed up the procedure. In cases where the test results in a higher than negative benefit, the test is allowed without a placebo, but always exceptionally.
Until they try vaccines they should solve other problems. For example, WHO is studying how to prepare the population to accept vaccinations. On the other hand, vaccines should be stored at a temperature of 80 ° C, which means that they will need special cold rooms in areas where vaccinations are to be carried out. And another aspect that worries is money. Who will pay the expenses? Neither the WHO nor the other institutions working with Ebola have doubts that Western countries will have to collaborate, but there is still no clear decision. However, WHO hopes they can start vaccinating before the end of 2015, overcoming all obstacles.
Buletina
Bidali zure helbide elektronikoa eta jaso asteroko buletina zure sarrera-ontzian








