Dmitri Ivanovitx Mendeleiev
1992/06/01 Azkune Mendia, Iñaki - Elhuyar Fundazioa Iturria: Elhuyar aldizkaria
Russian chemist born in Tobolsk (Siberia) on February 7, 1834. In his family were many brothers (it is said that they were 14 or 17, because the archives have nothing clear), being Dmitri the youngest. His mother was Mongolian and his father was director of the Institute. The grandfather, for his part, had the first printing press in Siberia and the first magazine was also published by him.
When Mendeleiev was very young, his father became obsessed and had to leave the profession of the Institute. Faced with the shortage of family income, his mother organized a glass factory. It is surprising that this woman goes forth to her family with so few resources.

On that occasion Mendeleiev heard the first chemical conference from a politician exiled to Siberia. At the end of his high school, in 1849 his father died and his mother's factory burned.
By that time all the brothers of Mendeleev had grown up and left the house. That is why the mother took care of the education of her youngest son. They went to Moscow, but there they did not succeed, as they could not access the university. From there they moved to St. Petersburg, where thanks to the help of a friend of his father, Mendeleiev managed to enter the university. His mother died shortly after studying.
Mendeleiev was the best student at the university. At the end of his career, he completed his studies in France and Germany. He worked as an intern in Heidelberg, worked with Bunsen and participated in the central congress in Karlsruhe. There he heard Cannizzaro ideas about the atomic weight of the chemical elements and was fascinated.
Back in St. Petersburg he was professor of chemistry since 1866. He was the wisest and most interesting speaker in Russia and one of the best in Europe. Between 1868 and 1870 he wrote the textbook The Principles of Chemistry, one of the best writings in Russian language and one of the best of all time.
Mendeleiev dealt with the different areas of science and technology. In addition to isomorphism, gas compression and air characteristics, he carried out work in agriculture, chemical industry and aeronautics among others. Also in politics he had his vicissitudes with the government. The authorities who spoke out against the oppression suffered by the students threatened several times.
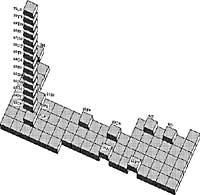
However, Mendeleiev’s most well-known and brilliant contribution to chemistry is his “Periodic Table of Chemical Elements”. Mendeleiev began to order the chemical elements by their atomic weight, from minor to major. Frankland immediately discovered something interesting about the properties of valence, explained fifteen years earlier. In Mendeleev's table the second element was lithium, with a valence, that is, it could be combined with a single atom. The next element in the list, beryllium, having two valence, could be combined with two different atoms. Then boron had three valence and then carbon four. The order was precisely 1, 2, 3, 4, 3, 2, 1.
Mendeleiev was able to order by atomic weights all the chemical elements known in his day (63 then), obtaining periodic increases and decrements of valence. He ordered the elements by rows, the lines between each other and the elements of the same valence in the same column. In addition, the elements of the same column or line are very similar to other chemical properties.
Because properties and balances rose and decreased periodically, the Periodic Table of Chemical Elements was referred to as the first published by Mendeleiev in 1869.
Mendeleev's table had a huge spread. For the first time he dared the work of a Russian scientist, unlike what was until then. The table in Russian was soon translated into German, leaving it in the hands of most scientists. The truth is that scientists did not immediately accept Mendeleev's work because until then attempts to order the elements failed.
Mendeleiev did not stop and his table was improving. On January 7, 1871, in the journal Journal of the Russian Chemical Society, he left gaps in the table for chemical elements still unknown, anticipating the chemical characteristics of these unknown elements. In 1875 Lecoq de Boisbaudran discovered the first element announced by Mendeleiev, Nilson and Cleve in 1879 the second and in 1885 Winkler the third.
Mendeleiev published in 1905 a textbook in which the advances made until then in chemistry are collected. By then he was a very popular chemist. He won many awards and in 1906 he was given almost the Nobel, since Moissan got it with a vote.
He died in St. Petersburg on February 2, 1907 and in his honor he was called mendelevio the chemical element 101 discovered years later (1955).

Gai honi buruzko eduki gehiago
Elhuyarrek garatutako teknologia