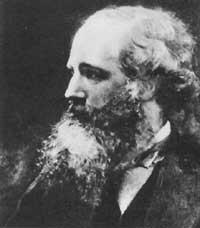Cavendish, Henry
1995/08/02 Azkune Mendia, Iñaki - Elhuyar Fundazioa | Kaltzada, Pili - Elhuyar Zientziaren Komunikazioa
(1731-1810)
English chemist who saw the light in Nice in October 1731. Due to his mother's poor health, the young Henry spent his first two years of life until his mother died and returned to London with his father. Cavendish was born in a noble and rich family that allowed him to live dedicated to research.

He studied at the University of Cambridge. However, without finishing the race, he left school and returned home from London. Cavendish was young, very shy. Before the questions of the faculty, he was silent and thus it was not possible to overcome the exams. He then began to show the tendency to monologue and to preserve it throughout his life. He did not speak with him, but with anyone, and one can say that he only lived until death took him. Therefore, his investigations came very late, until Maxwell collected and published the manuscripts of Cavendish.
In 1776 Cavendish reported on gas obtained by reactions between metals and acids. The truth is that Boyle and Hales had already carried out this experiment, but Cavendish was the first to systematically analyze the characteristics of the gases. That is why it is said invented by Cavendish. This gas was called hydrogen by Lavoisier. Cavendish studied gas behavior between 1780 and 1790. Among other things, he observed that after burning this gas was transformed into water. Water, therefore, was a mixture of two gases and not a single substance against what was thought until then.
In addition, the first experiments to determine its density by measuring the volume of gases are due to this researcher. In 1785 it produces a mixture of nitrogen and oxygen that dissolves the acid produced in water. Added more nitrogen to this mixture in order to completely remove oxygen. However, there was always a small amount of gas that seemed unmistakable. This was Argon gas, as Ramsay confirmed 100 years later.
In 1798, using the torsion scale, he measured the average density of the earth. According to Newton's law of gravitation, the average density of the Earth could be calculated by the force of attraction between the Earth and any object, but for it it was necessary to clarify the constant G. Knowing the contribution force between both objects, the constant G could be calculated theoretically. To that gave Cavendish the next years. The torsion scale was used, placing metal balls of the same weight at the ends of the shaft. Changing the weight of these balls, the axis of the torsion balance was inclined, being this diving the measure of the contribution force between the two balls. As he knew the mass and distance of two balls, he used Newton's equation to calculate the mass of the Earth. Since then this experiment has been named Cavendish.
Cavendish was accompanied by the science that only a friend had during his life. He died in London in 1810.

Gai honi buruzko eduki gehiago
Elhuyarrek garatutako teknologia