Building the clock of life

American Libby participated in the Manhattan project when she came up with a new use of carbon. During World War II, the project brought together numerous scientists with the aim of developing the atomic bomb as soon as possible. Libby had to find a way to separate isotopes from uranium, which passed nights and days. In the end they knew how to do it and developed the first bomb for 4 years.
However, Libby thought he was working on that work, another technique that will then bring him fame all over the world. In fact, in addition to uranium-235, he suspected that on Earth there could be radioactive substances of various origins, that is, that in addition to the radioactive atoms created with the planet, they could also have been created later, in continuous formation. In short, the Earth is suffering constant bombardment of cosmic rays and thought that these radiations could produce radioactive isotopes.
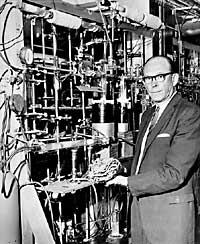
Libby quickly demonstrated what was planned, discovering tritium, a radioactive isotope of hydrogen. But it was not enough. It is estimated that there should be more isotopes, another isotope produced by gamma cosmic rays. He calculated that colliding cosmic neutrons with nitrogen atoms in the atmosphere would produce 14 carbon and hit the low isotope 14 C. The American discovered that a small percentage of atmospheric CO 2 was composed of 14C. By then it was already thought that this isotope could have a very attractive archaeological use.
Radioactive living beings
Libby began studying the living beings of the area and discovered that the isotope 14 C of the atmosphere settled on the plants. During photosynthesis, plants collect carbon dioxide (CO 2) and release it into respiration; during this photosynthetic breathing process, the chemist discovered that plants were continuously receiving and releasing 14 C radioactive in the atmosphere, that is, in plants the same proportion as the 14 C isotope in the atmosphere, in animals that eat plants and in other animals that eat animals was repeated. In all living beings, therefore.
The most interesting thing is that when living beings die their carbon exchange with the atmosphere breaks. Therefore, organisms after their death do not renew carbon 14. And that's when the 14C clock comes into operation! In fact, the 14 carbon, like the rest of radioactive isotopes, is unstable and slowly begins to disappear from the organism from the very moment of death, until it practically disappears in very ancient fossils.
The amount of 14C of the deceased decreases exponentially, rapidly disappearing at first and increasing over the years. Libby himself estimated that in the year 5,568 any fossil loses 50% of its 14C and that, after 55,680 years, still retains 0.01% of what was alive.

Based on this, comparing the 14 carbon ratios present in the fossil and in the atmosphere, he indicated that the age of any fossil could be determined. He explained this in 1949 to scientists at the Metropolitan Museum of New York and the University of Chicago. He calculated the age of some bones with the new method and, in view of the result, had to accept the validity of the method.
The use of 14C was extended year after year among archaeologists, to become the most used method. The dating system was then unique and also very reliable. So Libby won the admiration of scientists and in 1960 he received the Nobel Prize in Chemistry. In fact, it served to date any material from living beings: wood, coal, fabrics, animal branches, bones, plant remains... That is, environmentalists could use them to investigate the characteristics of ecosystems or anthropologists to analyze the facts of a society. It does not matter the size of the sample, where and how it has been maintained, or the reasons for death. Therefore, it was considered suitable for comparing samples from anywhere in the world.
Partial clock

The greatest limitation for the moment is dating from 50,000 years, since in the fossils of the hour there are 14 C below, which would require very sensitive devices to calculate exactly how many isotopes there are. Therefore, it is not useful to study phenomena such as the evolution of species, the formation of continents. Carbon 14 is like a watch with only a second needle and scientists often need hours, days and months.
The evolution of man himself can only be partially followed by carbon. In the Neanderthal bones there are only 14 carbons. Thus, other methods are used in Paleontology: on the one hand, radioactive isotopes that ‘go out’ more slowly, among them uranium-238a, and on the other, chemical analyses of bones, the study of pollen, the dating of sediments around bones, archaeomagnetism and thermoluminescence. In fact, combinations of all these methods are currently used to obtain reliable datations.
Carbon Calibration 14
Although the method is widely used in Archaeology, many believe it is necessary to calibrate it better. In fact, Libby did not accurately calculate the disintegration speed of 14C. It has subsequently been calculated that the mean life of the isotope is 5,730 years and not 5,568, as Libby calculated in 1949.

On the other hand, when indicating that the amount of atmospheric isotope 14 C is constant, it did not take into account that the formation of the isotope depends on the neutrons that reach the atmosphere and that the radiation that reaches the Earth is not constant at all. It is to be assumed that throughout history there would be notable variations in the proportion of 14C in the atmosphere and that living beings from one time and another would receive in different amounts.
Chemicals have found that in volcanic places the rate of 14C is itself lower, as the carbon released internally by volcanoes reduces the proportion of radioactive carbon in the atmosphere.
Moreover, throughout history there have been many events that have altered the amount of 14C. For example, it has been observed that carbon dioxide emitted into the atmosphere by industry since 1900 has considerably reduced the proportion of 14C. And yet the atomic experiments of the 1950s have grown again. Consequently, scientists believe that in 6200 BC the rate of isotope 14 C was 8% higher than the current one, so a fossil that claims that the carbon method is 7,500 years old can actually reach 8,500.
Scientists know that this is a better calibrated method, but the debate continues to serve to acceptably approximate the dating method of 14C.
Buletina
Bidali zure helbide elektronikoa eta jaso asteroko buletina zure sarrera-ontzian











