Trip inside the café
2014/06/01 Omar, Jone - Kimika Analitikoa Saila, Zientzia eta Teknologia Fakultatea, Euskal Herriko Unibertsitatea Iturria: Elhuyar aldizkaria

Coffee throughout the history
The history of coffee is very old. It began centuries ago when a pastor was struck by the special attitude of some goats. The shepherd saw that the animals ate round fruits of the bushes, took a handful of fruits and went to the wise man of the village. They were tasted and, desperate that they had no flavor, they threw them into the fire. As if a miracle had occurred, from the flames came a charming smell. It was the first footprint we currently have on the most consumed drink worldwide.
The coffee plant has its origin in Africa, specifically in Madagascar, tropical zone, whose cultivation began in the fields of Ethiopia and Yemen, where the climate and geography are quite similar. Initially, this drink helped keep people frozen, so the Muslims wanted to ban it thinking it was a contagious drink, but its consumption spread quickly. A century later (XVI. In the twentieth century the first coffee shops of Constantinoplan were established and later the first coffee house was inaugurated in London. They tried to put big taxes to put limits on consumption, but the French have already extended the drink to all Europe. Coffee is, therefore, the result of old customs and endless tests, and with this research work, it has wanted to build a level more in the history of coffee in order to be able to undertake the path.
Characteristics of coffee
There are two species of coffee, Arabica and Robusta. The type of Arabic grows on slopes in a climate of high humidity, while Robusta is the African continent where it grows the most. However, there are currently numerous mixtures between both species. Coffee beans once collected, cleaned, selected and dried are green, without smell or flavor. In the process of roasting the smell occurs, the color is changed to the coffee bihias, its size is increased... It can be said that the chemistry of coffee is quite complex as various physical-chemical processes occur inside the coffee bean.
Chemistry of coffee roasting
The smell of roasted coffee is formed by a complex mixture of volatile compounds, while non-volatile compounds determine their acidity, bitterness and astringency. Among the volatile molecules that make up the smell of coffee we can find several families: furans, pirazines, ketones, alcohols, aldehydes, esters, pyroroles, thiophiles, lactonas, alkenes, acids and oxazoles; to date 850 volatile compounds have been identified. The final taste of coffee can be influenced by different characteristics: coffee species, origin, conditions of the land in which it has grown, storage, time and tueste temperature. The latter, the roast, is the most important step in the whole process, since it directly affects the physico-chemical characteristics of the grain and determines the final smell and flavor. The research has brought together sixty-six types of coffee from different countries around the world (Brazil, Cameroon, Costa Rica, Nicaragua, Ivory Coast, Colombia, Indonesia, Ethiopia). They have been toasted at different temperatures and an analysis of the smell of the same has been carried out, as well as an analysis of the color variations and size of the coffee beans. But the most striking and, in general, the one that distances itself from the human senses is the interior of the coffee bean. Therefore, the coffee beans have been cut in half and the changes in the interior have been analysed in the different conditions of tueste.
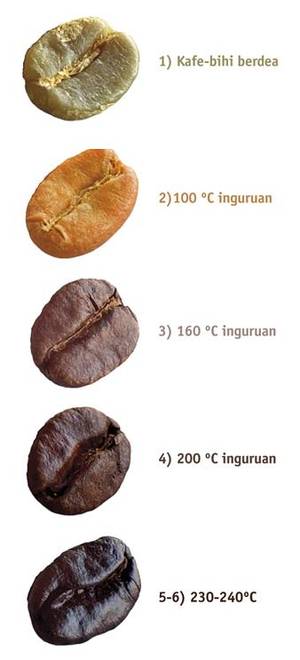
In general, the process of roasting coffee beans can be divided into 6 steps depending on the temperature used. In this research work we have tried to follow the same steps in order to better understand the internal chemistry of coffee:
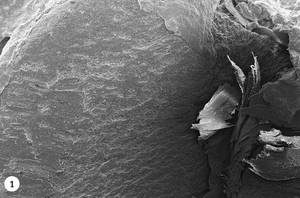
1. Green coffee beans are introduced into the roast drum. At first, the temperature will decrease due to the entry of the new product and gradually will increase again. An endothermic process by heat absorption is initiated, but from the 50ºC the first changes in the tissues can be observed. Photo 1 shows a photograph made using the SEM (Scanning Electron Microscope) technique, in which a piece of green coffee has been cut in half and its interior can be analyzed. In it we see a homogeneous structure that goes from the surface of the coffee bean (left) to the heart of the coffee bean (right).
2. As it is heated, the coffee bihias absorb heat and water is released in the form of steam. At 100ºC the reaction Strecker begins, where the amino acids are degraded and the sugars are caramelized, so the color of the coffee bean changes from green to yellow. The smell also begins to change, from being grass to being bread.
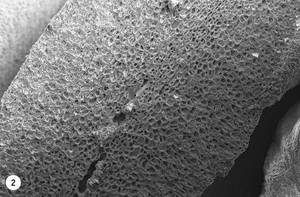
3. At 160 °C is produced the reaction “Maillard”, with the appearance of volatile compounds that will continue throughout the process of tueste. It increases the volume and begins the vitreous transition, appearing pores in the internal structure. Due to the high pressure generated by evaporated water and the high amount of gas produced, the cellulose wall is broken. This pore structure makes the gases escape outside and the oil migrates to the surface of the coffee bean. Due to the heating process (160-170 °C), coffee beans are darkened and the CO 2 produced half breaks the coffee bean. As can be seen in photo 2, several pores have already appeared, many of which have not yet been drilled, but at the bottom of the image is also seen the breakage of the coffee bean.
4º A 200ºC the reaction goes from being endothermal to exothermic, where heat is released and the coffee bees acquire a typical brown color and a complex flavor and aroma.
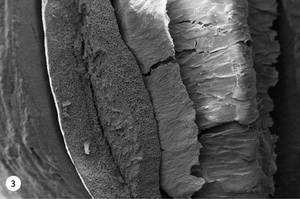
5. At 230 °C, the bihias of coffee acquire a stronger and bitter taste. Small drops of oil appear on the surface of the coffee bean that give it shine. At this temperature, the oil drops and the expelled gases have completely drilled the coffee bean, as shown in figure 3. The entire interior is full of holes and the central fissure divides the coffee bean.
6º From 240ºC, the surface of the coffee bean is covered with oil and the coffee begins to burn.
The volatile compounds that are generated at each tueste temperature by means of gas chromatography in different types of coffee worldwide have been measured. In addition, the value of the color has been measured, the empty volume (quantity of pores) that is generated in each grain, as well as the weight loss that the coffee clouds suffer. The objective is to know if there is a correlation between these physical-chemical changes that occur as a result of the increase in temperature. The growth of volatile compounds such as 2,6-dimethylpirazine, 2,3-dimethylpirazine, 2-acetylfuran, weight loss and the value of the empty volume inside the coffee beans are related to each other, while they are inversely proportional to the color change, since the darker the color is, the closer to one. Therefore, the physical changes that can be observed in the tueste of coffee beans have behind a chemical reason that has been able to be analyzed and understood more thoroughly with this research.
To deepen the idea of the migration of gases and oils, an elementary analysis of the coffee masses has been carried out. To do this, it is necessary to remove a new photo of the coffee rods previously cut, using the SEM technique, but this time coupled to the X-rays. This combination allows to analyze the dispersion of the inside elements of the coffee bean (Ca, O, N, C, etc. ). In green coffee beans, the elements are homogenously dispersed, but as the temperature of the roast increases, the amount of C and O increases in the center of the coffee bean, which reinforces the theory of gas migration. Pyrolysis and chemical transformations have led to high pressures, releasing a lot of CO 2 and breaking coffee in half. On the other hand, it has been commented that at 200ºC the reaction becomes exothermic, so in the central zone of the coffee bean accumulates a great heat that, as a consequence of the calcination, increases considerably the concentration of C.
With this work it is intended to make known the chemistry that hides inside the daily coffee. The color and smell of coffee beans are characteristics that we perceive when preparing breakfast coffee and that, if exercised a little, would allow us to differentiate at a glance the types of coffee. However, this journey into the interior indicates that there is still a lot of unknown information, and it highlights the importance of chemistry in a current daily product, since without these reactions and chemical processes we did not achieve an odorous treasure.
References References References References
Thank you Thank you!
I would like to thank the University of the Basque Country (UPV/EHU) and the Basque Government for the opportunity to carry out this project. My sincere thanks to Nestor Etxebarria and Maitane Olivares for having taught not to resign.

Gai honi buruzko eduki gehiago
Elhuyarrek garatutako teknologia