Hope to forget Alzheimer's
2017/09/01 Astiz Zurutuza, Xabier - Farmazialaria Iturria: Elhuyar aldizkaria
 400
400
Alzheimer's is the most common type of degenerative dementia. It is supported by elderly people and attentive to intelligence functions hindering daily activities. The damage is persistent and progressive, limiting the patient's autonomy over time. Finally, as a result of the damage caused to the organs necessary for life, the patient leads to death.
Unfortunately, today there is no way to cure Alzheimer's. The pharmacological and non-pharmacological treatments used only serve to reduce the evolution of the disease and improve the quality of life of the patient and his environment.
Now, however, the experimental drug Aducanumab opens its doors to the hope of finding a treatment that can definitely cure Alzheimer's.
About the origin of Alzheimer's
Although the origin of Alzheimer's is not yet clear, the hypothesis of b-amyloid (bA) is considered the main reason. According to this, the accumulation of bA proteins in the head produces extracellular and internal plaques that are toxic to the brain. This is probably because the body produces this protein disproportionately or cannot be eliminated.
An important risk factor for Alzheimer's is genetic. It seems that in the apolipoprotein E gene of chromosome 19 is one of the origins of the disease, since its fourth allele (ApoE4) is related to the production of < A. But there are other risk factors associated with the appearance of this type of plaques in the brain, such as age, lifestyle, anti-inflammatory abuse, or aluminum.
Aducanumab
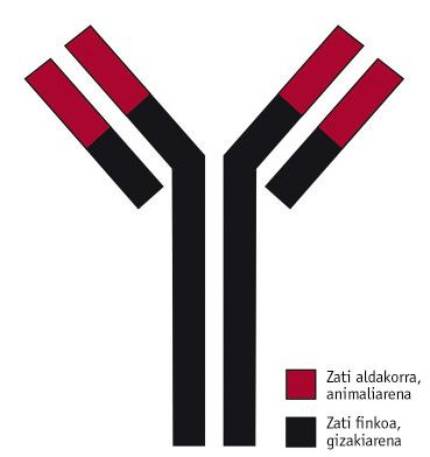
The drug aducanumab was developed by the American association Biogen. It is a selective monoclonal human antibody type IgG1, opposite to < A (Figure 1). The variable part of the drug is obtained from the rat and the fixed part obtained from a patient who suffered a particularly prolonged case of Alzheimer's. The first will attack the antigen (in this case at < A), while the part of human origin will serve to prevent the patient from suffering rejection of the immune system.
According to scientists, although Alzheimer's is diagnosed at the time of the onset of symptoms, < A plates have been stored in the patient's brain for years. That's why they believe that all the tests done so far with highly developed Alzheimer's patients have failed. Now, however, new clinical trials are being conducted with patients with low and medium degree of disease, such as those with aducanumab. The drug is still in development, but the creators have shown that it removes < A plates from the brain of these patients. If research is successful in the future, the goal of trials is to detect plaques very early and the doctor can prescribe them as any other medication.
First results
Two years ago Biogen launched the phase 1b clinical trial called PRIME. For one year, monthly aducanumab injections were given to participants and wanted to know their efficacy, safety and pharmacokinetics.
This involved 165 patients, some with placebo and others with different doses of drug (1, 3, 6 or 10 mg/kg). The influence of customs agents was measured by Positron Emission Tomography (PET).
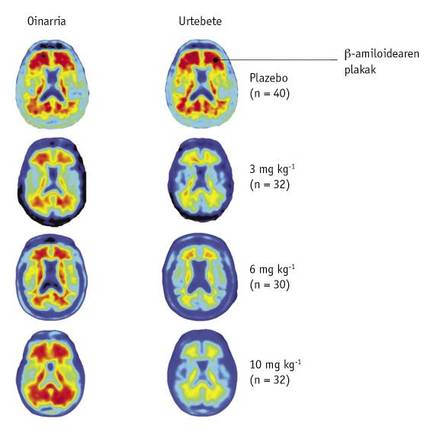
After one year, the PET images of patients were compared with the pre-treatment reference. In this comparison, scientists observed that the decrease in levels of < A was clearly seen according to the dose of the drug (Figure 2). The images also showed that this decline occurred in all parts of the brain.
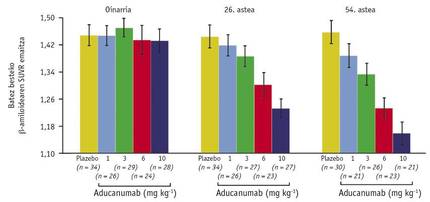
Another of the results obtained with this scanner showed a new satisfaction: the scale between the dose used and the average amount of < A in the brain (Figure 3). Through it, levels of < A of weeks 26 and 54 could be compared with the reference and concluded that the effect of the drug depends on time and dose. This discovery can be considered historical, as it suggests that for the first time Alzheimer's disease can be cured. And if all this was not enough, patients who received a dose of 10m/kg in Week 54 had a level of <A 1.16 very close to the limit of 1.10 that discriminates positive and negative results.
The methods called “Mini Mental State Examination (MMSE)” and “Clinical Dementia Rating (CDR)” were also used, two scoring methods used to detect, record and analyze the evolutions of cognitive impairments. In these methods the patient must complete a questionnaire and depending on the score obtained, the doctor is aware of the degree of dementia of the patient. The response time to all questions ranges from 5 to 10 minutes, so dementia levels are frequently analyzed. Using MMSE and CDR in this phase 1b, they were able to verify that decreased levels of < A report benefits.
In terms of safety, Aducanumab can be considered a fairly safe drug. The most common adverse effects were headache, urine infection, respiratory tract infection, and so-called ARIA (Amyloid-Related Imaging Abnormalities). The latter are anomalies found in magnetic resonance imaging, caused by edema, microhemorrhages, and siderosis. Although patients who suffered all these adverse effects were treated in the emergency room, no one has been hospitalized. There were no deaths either.
Finally, the customs pharmacokinetics (maximum concentration and surface under the normal curve) were linear at doses in all treated persons. The biological half-life in plasma is twenty-one days. On the other hand, the antibody against aducanumab alone developed 3% of the total patients. And since the influence of antibodies was transient and through the minimum antibody titers, they concluded that they do not affect pharmacokinetics and safety of adducms.
To continue these results, the three phases of the clinical trial were launched.
Final phases of clinical sessions
The third and final phase of the clinical trial is called EMERGE and ENGAGE. They started in 2015 and will last seven years. Its objective is to check the findings of phase 1b and continue the study of the efficacy and safety of aducanumab in people with a low and medium Alzheimer's degree.
To do this, they will hold sessions with 3,000 patients from 400 hospitals around the world. But it is not for any patient to participate in the research. The patient must meet a number of criteria in order to participate in the tests: That the PET scanner has a positive result of < A, allow the identification of the genotype ApoE4, a score between 24 and 30 in the MMSE method and a result of 0.5 in the CDR method, among others. Patients with a medical history of unstable angina or myocardial infarction, anemia, or chronic liver or kidney disease may not participate in the tests.
In this phase, as in the previous phase, different doses of antibody will be injected once a month and in 2022 the results of PET, CDR and MMSE will be compared with placebo.
Tracking from pharmacy
Like any other drug, we can also conduct pharmacotherapeutic follow-up from the pharmacy with aducanumab.
It is a service offered in the pharmacy office that aims to improve the quality of life of the patient. This is intended to increase the efficacy and safety of patient treatments, reducing the risks associated with the use of drugs. Pharmacotherapeutic follow-up is carried out following a specific methodology or systematic, for which it is essential to incorporate a pharmacist to the multidisciplinary healthcare team that serves the patient.
In order to carry out the service it is necessary to maintain regular interviews in the pharmacy with the patient or his or her caregiver. And in these interviews we can realize the small health problems that aducanumab can cause to the patient. It should be noted that Alzheimer's patients are elderly people and patients with multiple pharmacological treatments, which considerably increases the risk of new health problems. This can cause harmful effects of aducanumab by the direct effect of the drug itself or by interaction with other medications. Once these health problems detected in the pharmacy have been registered, they would contact the doctor participating in the clinical trial. We would also contact your family doctor if necessary. To end the cycle, when patients leave the medical consultation, a date for a new interview would be defined.
Thus, pharmacists can participate and collaborate in this third phase of the trial to make aducanumab the ultimate drug against Alzheimer's in the future.
Finally, it is worth mentioning that these meetings in pharmacy will serve to continue carrying out pharmacokinetic follow-up to the patient in the future. In addition to all the information we have recorded, relationships with the patient and with doctors from different fields will be closer, always for the benefit of the patient.
Bibliography

Gai honi buruzko eduki gehiago
Elhuyarrek garatutako teknologia