What do you wash with every day?
2002/08/18 Mendiburu, Joana - Elhuyar Zientziaren Komunikazioa
Formerly only ‘Chimbo’ or ‘Soap of Marseille’ was known. The body, clothes, hair, dishes… everything was cleaned with the same soap. At present they are found in all types of structures, colors and odors, but with the same base; fatty acid (animal or vegetable) and an alkaline substance (not acid). Additional products that differentiate one soap from the other: perfume, antiseptics, oils, etc. They are.
History of Soap
The use of soap comes from antiquity. In Egypt, Greece and Rome soap was produced with animal fat and ash of sweet aroma plants. Soap making techniques are believed to have evolved very slowly. One of the first changes was the substitution of animal fat by vegetable fat and olive oil.

XII. In the nineteenth century, soap production was very common in the area of Spain and Italy, due to the ease with which raw materials were obtained. In fact, the Italian town of Savone is the origin of the soap we know today.
In France, soap production began a century later. The first factory was built in the city of Toulon and, after the success obtained, the soap industry expanded in Marseille. XVIII and XIX. With the contributions of French chemicals over the centuries, techniques would come to understand the structure of soap and improve its manufacture.
Soap is a product with a large industry today, with a production of more than six million tons per year, but with a base difference between soaps not very high. The difference between the flexible and rigid structure, for example, is the alkaline used to make soap. Liquid soaps are based on petroleum derivatives and transparent soaps are added glycerin or alcohol. Finally, soap powder, which after dehydration has become ashes, is only soap.
The traditional function, the traditional structure

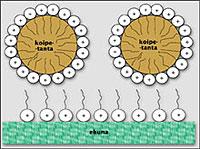
The soap owes its cleaning capacity to its peculiar structure. It is a bridge between water and dirt. In fact, soap molecules have two extremes: one is associated with fats that form dirt and the other to water. In fact, being unable to dissolve fats in water, it is possible to remove dirt with the help of soap.
For proper cleaning the soap is rubbed and the foam is formed. But what happens when no foam is produced? For example with sea water? In these cases the guilt is not of soap and it is useless to throw a lot of soap. The blame is for the water. In fact, with abundant mineral water the soap generates rainfall and does not generate foam. This water is called ‘hard’ water and contains mainly minerals of calcium and magnesium. On the contrary, if the domestic water is very poor in minerals, the foam will be easily achieved.
The cleansing effectiveness of soap is measured based on criteria such as dead cells, dirt, bacteria causing bad odors and ability to remove excessive sebum. It should be noted that the fact that soap generates many foams does not necessarily imply a good wash.
What soap to choose?

As has already been indicated, although the basic structure of the soaps is the same, there is nothing more to look at the shelves of the shops to lose among soaps of so many colors, textures and smells and forget that it is to clean the soap. Because they clean all.
However, in recent years the idea is spreading that soap is bad for the skin. This idea is based on the alkaline pH of the soaps. It is calculated that the average pH of the soaps is of the order of 10, while that of the skin is more acidic, of the order of 5.5. In view of these pH differences, it is normal to think that soap dries the skin (I would not put my finger in a pH 10 solution) and from that idea soaps of low pH are preached. We have been using soap for years, but the soap still has mysteries. However, it is true that the higher the pH of the soap, the more it will clean and the more it will dry the skin.

As a result, cleaning products derived from oil are currently sold and are called detergents to separate them from natural soap. But don't think the reason to develop the new product is the damage it can cause to the skin, let alone! This development, like others, is a direct consequence of the war. Precisely because of the hunger in World War I, the fat needed to produce soaps did not exist and the Germans looked for a product to replace soap. And get it! With the end of the war, the product stopped improving and we had to wait for World War II to perfect it. The relay was taken by the Americans, the detergent also with hard water, like sea water, which extracts foam. Thanks to this property was a suitable product for the soldiers of the navy.
On the other hand, there are other concerns related to multiple use of soaps. In recent years research has been carried out on new infectious diseases, highlighting that instead of killing bacteria, an opposite effect is occurring. That is, because of the excessive use of soap, more and more substances that cause infections are appearing. To prevent this from happening, it is considered that in normal conditions conventional disinfectants (soap and brush) are sufficient and that the hardest products should only be used in critical conditions.
Published in 7K.

Gai honi buruzko eduki gehiago
Elhuyarrek garatutako teknologia