Stem cells for neurodegenerative diseases
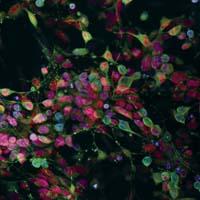
Neurodegenerative diseases are characterized by their involvement in certain neurons or neuronal groups, with the consequent progressive loss of ability to perform their function. Alzheimer's and Parkinson's are the most common. Currently, most existing treatments affect symptoms and clinical signs, do not affect the source - in most cases - or alter the pathogenic process. That is why we believe that to develop effective therapies, which can slow or restore neurodegeneration, it is necessary to generate knowledge. We must know the biological processes behind, sequentially identifying the targets and molecules that will interrupt or weaken the degenerative process.
Human stem cells allow us to investigate the normal and abnormal development of human neurons, both when isolated and when accompanied by other important cell populations. We can also investigate the factors involved in the selective vulnerability of neurons, since these diseases are characterized by influencing certain types of neurons: dopaminergic in parkinson, motor neurons of the front branch in amyotrophic lateral sclerosis... And finally, we can explore specific ways of survival and cell death.
In addition, the use of stem cells allows to design predictive models in vitro that allow analyzing and evaluating new drugs. These models allow testing drugs capable of influencing disease development and restoring the affected function. Finally, starting from pluripotent human cells, it is possible to create neuronal populations suitable for cell therapy in some diseases.
Reprogramming
For two years a new technology, cellular reprogramming through certain factors is available. It is a technique devised by Shinya Yamanakana that allows us to create pluripotenite cells or iPS (induced pluripotent stem cells) from adult cells differentiated from anyone (for example, superficial fibroblasts). The technique is based on transcription factors that maintain a state of pluripotency. Some transcription factors (proteins that regulate gene expression) are able to activate their corresponding genes and reactivate the molecular circuit that controls the pluripotency and self-innovation of stem cells. This type of reprogramming is based on the exceptional expression of these factors.
To integrate these factors into adult cells we use viral vectors. They transport transgen and markers as fluorescent proteins to be able to follow the process in vivo. The effectiveness of the process is low, but we are able to obtain genetically identical pluripotent cells from patients. Then we can work with these cells (in our case neurons): we can analyze in vitro what are the factors causing the onset and development of the disease and measure the influence of potentially therapeutic molecules. In the coming months, Inbiomed will have the necessary equipment for fluorescence directed cell distribution (FACS), which will allow these studies to be carried out more quickly and accurately.
Cell reprogramming has meant a revolution in the field of stem cells, eliminating some ethical and social problems related to the use of embryonic stem cells and fetal tissues. However, it should be noted that this strategy is valid primarily for diseases of genetic origin, since other factors (epigenetic) are "eliminated" in the reprogramming process. So, for the moment, two types of pluripotent cells (embryonic and reprogrammed) are complementary. On the other hand, it should be noted that these models of diseases have at the moment great difficulties in reproducing the influence of age and aging (critical factors in the development of neurodegenerative diseases).
Alternative cell therapy
A group of neurons that produce dopamine die in Parkinson's disease. Therefore, the amount of dopamine decreases in an area of the brain that modulates movements and motor symptoms characteristic of the disease appear. The administration of the precursor or through drugs that mimic its effect eliminates the symptoms of the disease.
Since the neuronal population affected by the pathological process is relatively known, alternative cell therapy - i.e. dopamine production by transplanting healthy neurons - was considered an option for more than 30 years. Cell therapy trials began in the late 1980s and fetal neurons have been used to vaccinate patients with Parkinson's disease. Transplanted neurons have been shown to have lived up to 14 years in many patients, including restoring adult brain function, but in other cases the benefit has been very limited or has not been beneficial, and some patients have also suffered damage. One of the biggest obstacles to the optimization of this type of treatment has been the shortage of appropriate cells, which has caused so much expectation in this field of research.
The fertility of adult stem cells is limited and the number of adult lineages they can produce is very small. On the contrary, pluripotent stem cells can derive any cell from an adult organism. The use of adequate growth factors and means allows the differentiation of stem cells towards dopaminergic neurons identical to those degenerated by the effect of Parkinson's. We hope that with these neurons we can create a source of cells suitable for therapy. For this, any drug must meet the requirements of safety, equality, capacity, repeatability, etc. ). Research is advanced, but it is impossible to say when it will become real patients.
I would like to convey an important idea to finish. In the development of research the relationship between investment (effort, time, money) and translational results is NOT linear. It is NOT correct, especially when analysed in the short term. This concept is very important, as it cannot be separated from the concept of research, and is fundamental to avoid false expectations that generate frustration and social discontent with scientists.
Buletina
Bidali zure helbide elektronikoa eta jaso asteroko buletina zure sarrera-ontzian








