Tumor angiogenesis
2002/02/01 Orive Arroyo, Gorka - Farmazian doktorea. Biofarmazia, Farmakozinetika eta Farmazia-teknologiako irakasle kolaboratzaileaFarmazia Fakultatea UPV-EHU, Vitoria-Gasteiz Iturria: Elhuyar aldizkaria
In the last 30 years, many research groups from many countries have dedicated their efforts to cure this disease. Yes, although it is one of the most studied and studied alterations from a biological and molecular point of view, there are still many doubts to solve and it is about to find the treatment of many types of cancer. In fact, the diversity of types of cancer makes the discovery of a ‘magic bullet’ or, what is the same, the development of a treatment against all kinds of cancer is virtually impossible. Therefore, the scientific objectives are directed, on the one hand, to the search for specific solutions for each type of cancer and, on the other, to the development of new therapeutic strategies that complement or replace the current techniques of surgery, chemotherapy and radiation therapy.
One of these new strategies against cancer is antiangiogenesis therapy. This strategy of increasing importance is based on the inhibition of the creation of new blood vessels that feed and dress the tumor (Folkman, 1971). In this study, in addition to affecting the importance of angiogenesis in the development of cancer, the therapeutic possibilities offered by their inhibitions will be analyzed.
What is tumor angiogenesis?
The factors involved in the creation and diffusion of cancer are numerous. These factors, both hereditary and epigenetic, modify the genetic material of the cells, especially concerning their reproductive capacity. Therefore, a cell that was previously divided in a controlled and limited way makes it infinite. However, the growth of cancer is not only due to cell immortality.
In prostate cancer, for example, the fraction of cells that are divided is only 2% (Berges, 1995), and 90-99% of the tumor cells generated die from lack of nutrients. What does this mean? That many types of cancer need a new development to keep the cell population developing. This phenomenon is angiogenesis.
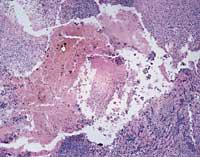
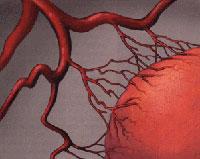
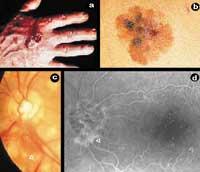
Angiogenesis, therefore, is the process of forming new blood vessels from the already existing ones. The new blood vessels, in addition to feeding the tumor, will allow its metastasis, that is, its extension to the new parts of the body. In order for a small town to become a city, like roads that communicate houses apart from the new ones, cancer needs, in addition to cells, blood vessels to feed, grow and expand. However, angiogenesis is not only a cancer-related phenomenon, but it is also a process that occurs in certain physiological situations (menstrual cycle and scarring) and physiopathology (psoriasis and diabetic retinopathy) (Figure 1).
Onset, phases and effects of tumor angiogenesis
The formation of new blood vessels is a reflection of the need for nutrients and oxygen of the tumor. When this need appears, tumor cells secrete substances called “angiogenic factors” or “positive regulators”. These factors affect the endothelial cells of the blood vessels and initiate angiogenesis.
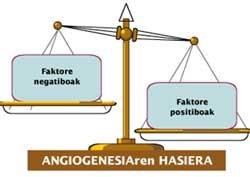
In normal conditions, the concentration of these factors is in balance with the concentration of others with adverse effect. The latter are called ‘antiangiogenic’ or ‘negative regulators’. Cancer, fostering the relative concentration of positive regulators that excite the formation of new blood vessels (Figure 2), breaks this balance.
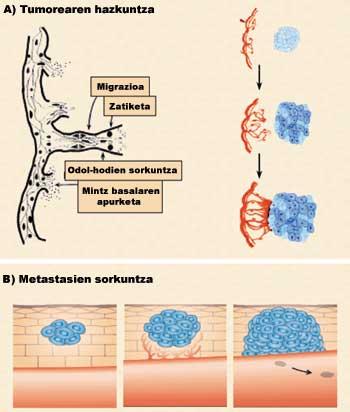
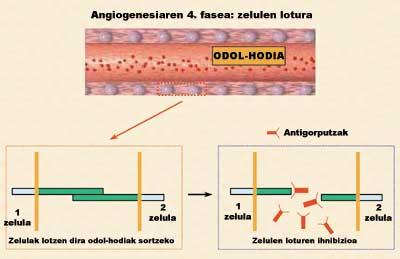
Angiogenesis is divided into four phases (Figure 3). In a first phase the basal membrane of the already existing blood vessels is broken (1st phase: break of the basal membrane). As a result, endothelial cells are expelled from the blood vessel and dispersed to the tumor area (2nd phase: migration). On this path the cells divide (Phase 3: Division). Finally, cells bind each other through different adhesive proteins forming a new blood vessel (Phase 4: cell junction).
The development of these four stages has two main consequences: on the one hand, tumor growth due to nutrients, oxygen and stimulating factors (figure 3.a), and on the other, the introduction of tumor cells in the blood vessels, which extend through blood to other parts of the body and cause new tumors (figure 3.b).
Inhibition of tumoral angiogenesis
Currently, the relationship between the vitality of tumor angiogenesis, the frequency of metastases and the reduction of the life expectancy of patients is known (Pluda, 1997). Therefore, the effective inhibition of angiogenesis has become the goal of many scientists. Above all, avoiding the formation of new blood vessels, since in addition to inhibiting metastasis, it will reduce the size of the tumor, facilitating the eradication of cancer by chemotherapy or surgery.
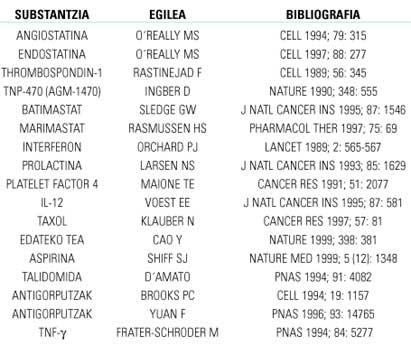
Taking into account the above, an easy way to inhibit angiogenesis would be to direct it towards negative regulatory balances of factors. This can be achieved in two ways: by inhibiting positive factors or promoting negative factors. Any phase of angiogenesis can also be inhibited if the enzymes and/or proteins involved are blocked. Table 1 shows the most effective drugs against angiogenesis investigated in laboratory animals. Of these, only those who best results and more security offer will try in humans with clinical sessions.
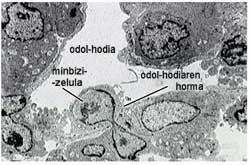
Of the four phases of angiogenesis, one of the most interesting is that which has been least studied from a therapeutic point of view. Therefore, the Pharmacy and Pharmaceutical Technology Laboratory of the School of Pharmacy (UPV/EHU) started a new study of angiogenesis inhibition to block this fourth phase. The aim of the research was to inhibit some adhesive proteins that allow the union between endothelial cells. To inhibit or block these proteins, cells that secreted specific antibodies were used (Figure 4). However, if cells were administered as such, it would quickly remove the animal's immune systems for considering them unknown.
To solve this problem, we group them into small polymeric spheres that protect cells against the immune system. This process is known as cell microencapsulation (Orive G, 2000). Within these small capsules, the cells, besides growing, secreted the antibody that interested us. This new anti-angiogenesis strategy has two main advantages: on the one hand, the inhibition of a protein little studied and on the other, the continued and prolonged secretion of antibody by cells.
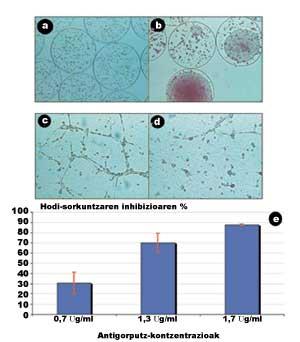
Figure 5 presents some of the results obtained in this study (Orive G, 2001). In the following images, obtained through the microscope, it is observed that cells grow and divide rapidly inside the capsules, resulting in the formation of violent aggregates (Figures 5.a and 5.b).
In another session, we analyzed the ability of secreted antibodies to block the union between endothelial cells. For this purpose, endothelial cells were stimulated by growth factors. These factors stretched the cells and facilitated the joints between them, creating immature blood vessels (tubules), that is, structures prior to the blood vessels that will feed the tumor. As shown in Figure 5.c, in the absence of antibodies, relations between endothelial cells are very remarkable.
On the contrary, when the dissolution of antibodies with a concentration of 1.7 µg/ml was introduced, the intercellular junctions and, therefore, the formation of tubules was almost completely inhibited (Figure 5.d). What is its meaning? That the antibody that secrete encapsulated cells has the ability to inhibit angiogenesis and that blocking of adhesive proteins is a good strategy to inhibit tumor angiogenesis. In addition, a last session showed that the relationship between concertistic antibody traction and the ability to inhibit angiogenesis is directly proportional (Figure 5.e).
These encouraging initial results have led us to a new research in which we will use gene therapy as a basis to inhibit tumor angiogenesis.
End of the End
Since the 1990s, angiogenesis has been becoming more and more interesting for both scientists and industry. In fact, more than 25 drugs against angiogenesis are being treated in clinical sessions (Kerbel, 2000). The current interest is focused not only on the discovery of new drugs, but also on the combination of antiangiogenesis strategies and conventional anti-cancer strategies (chemotherapy, radiation therapy, and surgery).
This last idea has great importance considering that the combination of several strategies can predict synergistic effects, that is, effects more beneficial than those achieved individually by such strategies. In addition, new administration strategies (monoclonal antibodies, gene therapy, etc. ). ), allow new solutions to inhibit angiogenesis and thus control cancers.
In short, although much remains to be investigated, daily work and the sweetest sleep of all scientists is to make their own the bad diseases.

Gai honi buruzko eduki gehiago
Elhuyarrek garatutako teknologia