Development of advanced recycled polymers: giving new life to plastic waste

This year has been 100 years since Hermann Staudinger published his first work on macromolecules, which began the science of polymers. Today, polymers, including plastics, are of paramount importance, as they are used in many fields, and even some advances would not be possible without these materials. However, when the 100th anniversary is celebrated, polymers face a great challenge: the large amount of waste generated by their use, which has generated great social concern. To overcome this challenge, in addition to reducing the use of polymers in general, scientists need to investigate new ways to recycle these materials and seek solutions to the problems and barriers that arise. That has been precisely the objective of this thesis.
Polymers: multifunctional materials
Today, polymers, including plastics, are used in multiple fields and are fundamental in everyday life. Polymers consist of repetitive units that are characterized by giant molecules of high molecular weight, macromolecules. These macromolecules are obtained by the reaction of small molecules, that is, monomers. Through an example you can easily understand: if the monomer is a clip, the polymer is a long chain formed by links between clips (Figure 1). There are several types of polymers that melt and solidify when cooled, and the process can be repeated over and over again.
Polymers have replaced various types of materials, such as metal, wood and glass, for their advantages of being cheap, light and easy to process, that is, they are easily shaped by dissipating little energy. XX. Since the first synthetic polymers appeared in the 20th century, several polymers have been developed to meet all application needs. Polymers can present a wide variety of properties, so they are used in multiple applications, mainly in packaging, medicine or renewable energies (Figure 1).
The problem of plastic waste
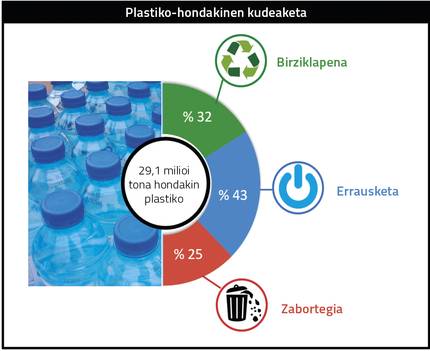
In recent years the use of polymers has generated a lot of debate and concern in society, since they generate a lot of waste and end up dispersed in the environment. According to data from the European Union (Figure 2), 29.1 million tons of plastic waste were generated in 2018: 32% were recycled and 43% were sent to incinerators to recover energy, while 25% were deposited in landfills. This data highlights the need to encourage the recycling of plastics, so it is absolutely necessary to investigate the recycling of materials and look for possible applications. This has been one of the objectives of this thesis.
Polyethylene terephthalate: bottled
If all industries are considered, packaging is the sector that recycles the most materials. Different polymers are used in packaging, including polyolefins, that is, polypropylene (PP), some types of polyethylene (PE) and polyethylene terephthalate (PET). PET is widely used in bottle making for its mechanical characteristics and chemical resistance. Given the importance of this material in this sector and the short life of use of these containers, the way to recycle them has developed a lot. Figure 3 shows the recycling process of PET, collecting the bottles and transporting them to the company, crushing, cleaning and drying them and finally proceeding to their processing, obtaining plates or threads.
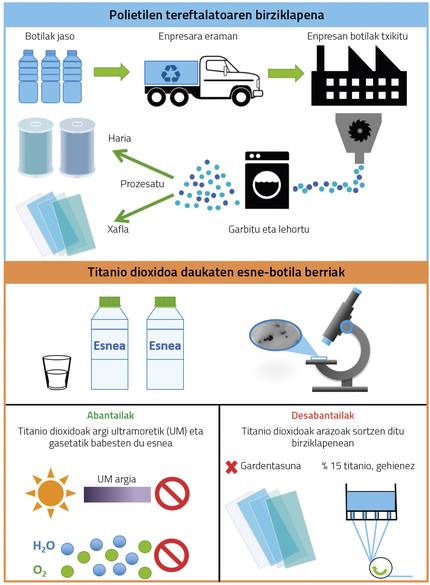
Currently in France, titanium dioxide nanoparticles (TiO2) are added to PET for the manufacture of milk bottles. In fact, titanium dioxide protects the contents of ultraviolet (milk) radiation and makes it difficult to filter gases. But these nanoparticles prevent material recycling. On the one hand, the incorporation of nanoparticles represents a loss of transparency, while transparent PET is the one that has the most demand in the market. Moreover, PET with titanium dioxide should contain less than 15% of recycled material, otherwise coiling problems occur (Figure 3). Consequently, in recycling plants, PET with titanium dioxide is distributed, which increases the process. It should also be noted that bottle caps are made of polyethylene or polypropylene, so before recycling it is necessary to separate the body from bottles and caps, which also makes the process more expensive.
Because PET/TiO2 cannot be recycled, it can be taken to landfills or incinerators to recover energy, but these options are not sustainable. From the environmental point of view it is a more sustainable option to recycle bottles with caps and the development of advanced materials, so that a non-recyclable waste can be used as raw material, giving a new life to the material.
Recycling the whole bottle: PET/PE/TiO2 mixing composite
The PET/PE/TiO2 mixing composite is obtained by recycling the whole bottle with stoppers. Although mass recycling is the most economical option, it is necessary to analyze the characteristics of this system if you want to give different applications. First, a mixture of composition similar to recycled material will be prepared, using commercial materials, which will be used to carry out the research. To know the composition of the recycled material, various techniques have been used, comparing the data with a bottle of French milk. Once the composition is known, the right quantities of commercial PET, PE and titanium dioxide have been added to the mixer. The material is heated to melt and mix.
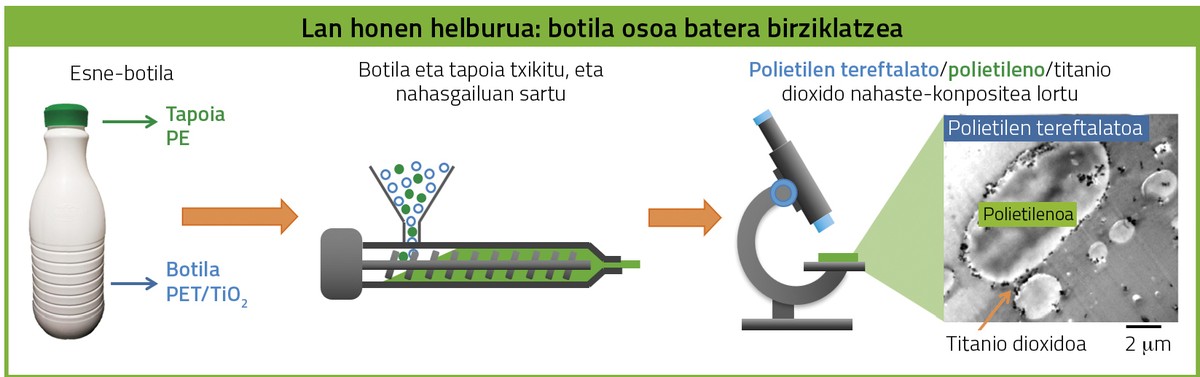
Once the mixing composite is prepared, its morphology must be investigated, that is, how PET, PE and TiO2 are organized. For this purpose, electron microscopy has been used. Thus, it has been observed that in PET/PE/TiO2 mixing composites the PE drops are dispersed by the PET matrix, as these two polymers are immiscible. Titanium dioxide is at the interface between the two polymer phases (Figure 4). TiO2 nanoparticles reduce the size of polyethylene droplets, which is beneficial as they improve their physical properties, especially mechanical ones.
The material's discharge capacity, i.e. the rheological response, has also been analyzed. The study of this characteristic is of vital importance, since it is necessary to heat the polymers and pour them properly so that they fill the mold taking its shape. This work has shown that nanoparticles have increased their emission capacity, so the processing of polymers will require less energy than PET processing.
Finally, some of the end-of-life properties of the material have been investigated. The permeability of some gases and vapors has been measured, among others. According to the results obtained, the PET/PE/TiO2 mixing composite has good barrier properties for oxygen and water vapor.
In terms of mechanical characteristics, the PET/PE/TiO2 mixture has an adequate stiffness, while in terms of break deformation it has a deformity higher than that of recycled PET.
According to these studies, the material resulting from recycling the entire bottle could be used for packaging due to its barrier properties and proper mechanical characteristics. In addition, it can be used for making interior plastic panels and other parts in cars for its mechanical characteristics.
Development of intelligent recycled materials: Joule effect
Beyond a step, intelligent materials have been developed incorporating conductive nanoparticles in the recycling process. Intelligent materials are materials capable of responding to an external stimulus, changing their physical or chemical characteristics. The thesis has used the heating effect of Joule, whereby when applying an electric current to a semiconductor material, the temperature increases.
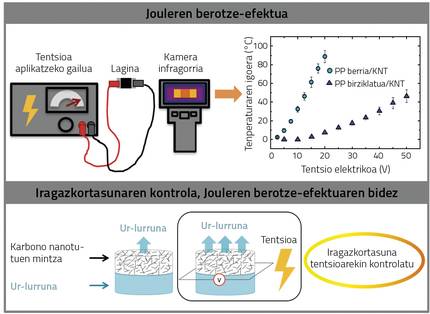
In this case, another polyolefin widely used in packaging, polypropylene, has been chosen. Both new and recycled polypropylene (PP) have been used and carbon nanotubes (KNT) have been added in order to obtain semiconductor materials. Figure 5 shows the system used to measure the Joule effect. An electrical device that applies direct current has been connected to the sample and an infrared camera has been used to measure the temperature. The samples have been given a different electrical voltage, obtaining the results shown in Figure 5. In the case of new PP and KNT, the temperature can rise to almost 90ºC by applying a voltage of 20V. In the case of recycled PP and KNT, on the contrary, the temperature increase is lower (for the reasons shown below): The application of 50V implies a temperature increase of about 40ºC.
According to laboratory analyses, recycled PP contains impurities, mainly titanium dioxide and talc residues and other inorganic compounds. These particles are not conductors and hinder the conductivity between nanotubes. However, the results show that a significant Joule effect also occurs in recycled PP.
Thus, it has been shown that a suitable way for obtaining semiconductor materials is the addition of carbon nanotubes to recycled polypropylene. These materials are intelligent, because when applying the electrical voltage they are heated and can have multiple applications, such as the use of the current as adhesive detached in one or thermal blankets. Another interesting application is the development of membranes, since permeability varies greatly with temperature, that is, changes the amount of a gas or steam that crosses a membrane. This has been the application that has been investigated in this work. Depending on the applied tension, we can control the temperature of the membrane. Thus, different permeability values can be obtained depending on the applied tension, as can be seen in figure 5. These materials can have applications in sensors or water desalination processes, among others.
Bibliography
Areizaga, J.; Cortazar, M.; Elorza, J. M.; Iruin, J. J. Pocket (Synthesis, Madrid 2002).
Comite Technique pour le Recyclage des Emballages Plastiques (Cotrep) (2015). “Note preliminaire 2. Impact du development du PET opaque blanc sur le recyclage des emballages en PET”.
El Tantawy, F. (2001). “Joule Heating treatments of conductive butyl rubber/ceramic superconductor composites: A new way for improving the stability and reproducibility?”, European Polymer Journal, 37: 565-574.
Iruin, J. J.; Elortza, J. M. Macromolecular Physical Chemistry (UEU, 1998).
Plastics Europe (2019). Plastics-The Facts. (Brussels, Belgium).
Roy, D.; Cambre, J.N. ; Summerlin, S.A. (2010). “Future perspectives and recent advances in stimuli-responsive materials”, Progress in Polymer Science, 35: 278-301.
Buletina
Bidali zure helbide elektronikoa eta jaso asteroko buletina zure sarrera-ontzian











