Ozone layer hole
2005/04/01 Gorrotxategi Zipitria, Mikel Iturria: Elhuyar aldizkaria
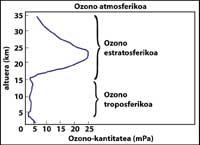
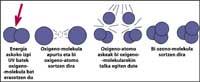
The ozone layer is located in the stratosphere, between 15 and 35 km, surrounding the entire planet. The ozone layer consists of ozone gas molecules. Each ozone molecule consists of three unstable oxygen atoms. Remember that oxygen molecules are made up of two oxygen atoms. The ozone molecule acts as a filter for ultraviolet radiation called B, that is, between 280 and 320 nanometers (called UV-B). UV-B radiation is harmful to animals and plants on the planet and, of course, also to humans.
However, ultraviolet radiation is essential for ozone formation, as ultraviolet radiation releases oxygen molecules. They react with other oxygen molecules to form ozone.
Effects of UV-B and ozone radiation on humans
Lack of stratospheric ozone is harmful to the animals and plants of the planet. But the presence of ozone at ground surface level is also harmful. This is because ozone is unstable, so it reacts easily with other chemical elements. Ozone makes breathing difficult, especially humans with asthma, and causes damage to trees and crops. Scientists have not yet clarified how an unstable gas such as ozone can be produced at the terrestrial surface level, nor why it occurs especially in the environment of large cities. Its inhabitants are already accustomed to the ‘ozone alarms’ as the Chilean capital, Santiago de Compostela.
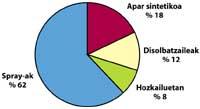
Human pollution or natural pollution?
It
is clear that chlorine (Cl) has reached the stratosphere, but is it due to human pollution or has it come to the stratosphere spontaneously? In fact, large amounts of chlorine can be found on the surface of the earth, such as sea salt (NaCl).
When the sea water evaporates also evaporates a quantity of salt, but returns to the ground with rain, ice or snow, because it is soluble in water. Another place where chlorine can be found in large quantities are swimming pools, but this chlorine is also soluble in water, so it does not present problems.
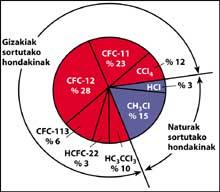
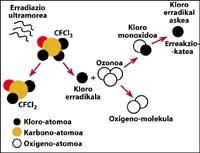
One of the sources of natural pollution are volcanoes, which throw hydrogen chloride into the atmosphere, but chlorine becomes hydrochloric acid, soluble in water. However, halocarbons used by humans, CFCs, carbon tetrachloride (CCI4) and methyl chloroform (CH-CCI3) are not soluble in water so they do not fall on Earth with rain, ice or snow. Therefore, they reach the stratosphere accompanied by wind. Given this, it is clear that stratospheric chlorine that destroys ozone is produced by humans.
How is the ozone layer destroyed?
It
is fully demonstrated that chlorine destroys ozone. This process occurs when UV radiation releases the CFC chlorine atom. The chlorine atom reacts and destroys with an ozone molecule, but after the reaction the chlorine atom remains free. It continues to react and destroy other ozone molecules. This process is totally harmful as a chlorine molecule can destroy about 100,000 ozone molecules. This process remains when chlorine is combined with another element. The average life of a chlorine atom in the stratosphere is about two years.
But it is known that this reaction occurs in the stratosphere and that a CFC molecule has more weight than air, so how is it possible for a CFC molecule to reach the stratosphere? This is because the wind is always moving and mixes the chemicals in the wind. This mixture and movement raises the CFC molecule with a force greater than it would fall with its weight. In addition, the CFC molecule does not react at Earth level or dissolve in water, so the CFC molecule is easily achievable with its weight in the stratosphere.
Why were CFCs used?
Once you read the above question, anyone comes up with the other. CFCs were created by General Motors chemists in the 1920s. The use of CFCs was very extensive and used in many industries. The reason is that getting CFC was quite cheap. In addition, it should be noted its stability, since the tests carried out showed that it did not react with other molecules. To this we have to add that it was not harmful as being. In addition, it does not dissolve in water. However, no one thought what was going to happen when this molecule reached the stratosphere.
Why at the poles?
It is clear that pollution does not occur at the poles, but thousands of kilometers away. Therefore, it seems very rare that the effects of pollution occur at the poles. But this fact is right.
CFCs are produced and released mainly in the northern hemisphere. In particular, 90% of the world's CFCs occur in the former Soviet Union, Japan, Europe and the US.
The CFCs then climb into the stratosphere using air currents in the tropics. Once in the stratosphere, they reach both poles taking advantage of the winds. Therefore, CFCs spread throughout the atmosphere. But there is a difference between the two poles: meteorology. The weather difference is based on the polar terrestrial surface, as the South Pole is a large area of land surrounded by sea, while the North Pole has a smaller terrestrial surface and the surrounding sea has large expanses of land, such as the islands of northern Canada, Greenland, Scandinavia...
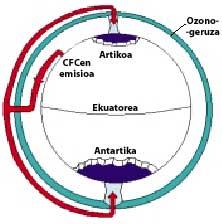
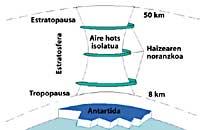
The weather of the south pole produces cool temperatures. In the six summer months there is light 24 hours a day and ozone absorbs UV rays. This causes a slight increase in temperature. However, during the six months of winter the air cools slightly, so it lowers the air and begins to rotate over Antarctica. The air speed rises to 400 km/h for spring. This swivel keeps the air inside, so there is no confusion with the outside air. So far there are no differences between Antarctica and the Arctic. But in the Arctic this swirl of air breaks several times due to the lands mentioned. Consequently, the inner air of the swirl is mixed with the outside.
In Antarctica, on the contrary, this whirlpool has no obstacles and the Arctic temperature is 10 or 15 times lower than in winter, that is, around 80 C below zero. In the Antarctic spring, with the first solar rays, the stratosphere begins to warm up and decrease the force of the air swirl. By mid-November this swirl disappears completely. However, at earlier cold temperatures, nitric acid produces stratospheric ice mists, causing a chemical environment favorable for ozone destruction.
In these mists, HCl and ClONO 2 react to each other by forming nitric acid and chlorine (Cl 2). This molecule, in principle, is stable and does not react with ozone, but solar rays photolyse it and decomposes it into two free radical chlorine that react with ozone. In the North Pole, higher temperatures reduce the generation of fog and therefore the destruction of ozone.
Will the ozone layer hole increase?
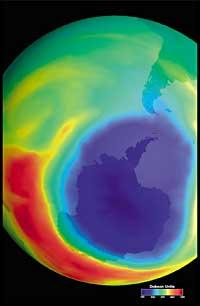
Lately there has been a fear: if the ozone hole will reach South America, the land closest to Antarctica. But before responding you have to know what is considered a hole for South America. Experts call it a hole when in South America the ozone column is less than 220 DU (Dobson unit). Given this, it can be said that the ozone hole has reached South America. In fact, in southern Chile measures below 220 UD have been taken. But this has only happened between September and October, and only for a few days. Therefore, the South American edge can be considered as the hole limit of the ozone layer.
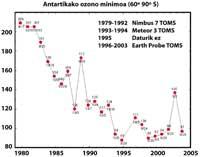
Scientists are hopeful for the future. In fact, CFCs are prohibited since the signing of the Montreal Protocol (1987). Taking into account the studies carried out, it is expected that within 50 years the CFCs will disappear, thus recovering the natural balance of ozone.
But all this data should be taken very cautiously, since it was thought that in 2000 the hole of the ozone layer reached the greatest extent in history and that in the following years it would not have increased. In nature, however, there are many elements to consider, as is the case that in 2002 no apparent hole was seen due to the heat of the year, but in 2003 appeared with its usual extension, being the second largest known.
However, it is true that the tropospheric concentration of ozone harmful substances has been declining since 1995. But harmful substances need between three and six years to move from the troposphere to the stratosphere. In recent years the concentration of chlorine in the stratosphere has remained constant and has begun to descend slightly. The future of ozone, however, depends not only on chlorine, but also on other substances used by man (methane, nitrous oxide, sulfate particles). And climate change.

In short, it can be said that among the substances present in the atmosphere are very complex processes and that it is difficult to take into account all the variables involved to detect what will happen in the future. But the data we have are hopeful, it seems that ozone destruction has stagnated and will improve gradually. However, it is clear that the future atmosphere will not be the same as it existed before the 1970s, and atmospheric substances should be carefully analyzed.
Damage to ultraviolet radiation It is now demonstrated that, in addition to clear and precise evidence, the increase in UV-B radiation has harmful effects for humans:
It affects the immune system. It hurts the eyes, for example, causing cataracts. It deepens sunburn and ages the skin. Increases the risk of allergic and toxic dermatitis. It strengthens some diseases of bacteria and viruses. Reduces fishing performance and harvests. |
How is ozone measured?
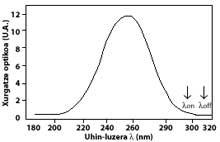
The most widely used technique is differential absorption. The technique is to measure the radiation of two waves coming from the sun to the earth's surface. The wavelength of these two waves is very similar, but at those wavelengths the ozone attenuation is very different.
Specifically, the lambda on SUB off is 315 nanometers, where the absorption of the effective ozone section is several times greater than that of lambda on en lambda off. In addition, the attenuation of solar radiation from other components present in the atmosphere in this part of the spectrum is very similar, so the division between the irradiation of two wavelengths accounts for the amount of ozone.
Specifically, it can be said that if we call I lambda the solar radiation of a certain wavelength that reaches the Earth, its value is:

where,
- L the length of the atmosphere crossed.
- Attenuation coefficient due to dispersion k M lambda Mie.
Solar irradiation I or lambda outside the atmosphere. Attenuation coefficient due to atmospheric components
K ab lambda (water vapor, ozone, N 2, 0 2, CO 2 ..).
k Attenuation coefficient due to dispersion A lambda Rayleigh.
For wavelengths around lambda onand lambda off, the value of the coefficients Rayleigh and Mie can be considered equal, i.e. k A lambda on = k A lambda off and k M lambda on = k M lambda off. Likewise, in this area of the spectrum, the absorption of atmospheric components is negligible, except for ozone. Therefore, the attenuation suffered by sunlight in this area of the spectrum is due only to stratospheric ozone. It is common that lambda on = 300 nm and lambda off = 315 nm and k or lambda on = 7 ko lambda off, that is, the absorption of lambda on is seven times greater than that of lambda off. Accordingly, the lambda on and lambda off intensity coefficient is obtained as:
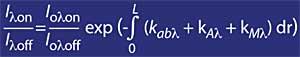
If the ozone absorption coefficient is defined by k or lambda, the ozone concentration (N) and its effective absorption section (T), the above expression remains as follows:
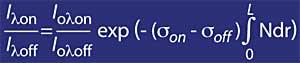
But it is necessary to take into account the air, for it will be used mr, where mr is the relative mass of the air. It should be noted that the mr factor is corrected according to solar time, day of year and latitude to make it reliable.

where,
- if D is the average width of the ozone layer.
- N is the average concentration of the ozone layer.
Consequently, it has been explained how knowing the measures I lambda on e I lambda off you can know the amount of ozone.
The arrows in the image on the left indicate where the differential absorption is performed. Lambda on = 300 nm and lambda off = 315 nm, where the absorption efficiency of ozone is several times greater than lambda on en lambda off.
Also, we have to take into account where we are and what day of the year is to know if the ozone measurement result is high or low.
Ozone layer hole history
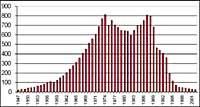
It was first discovered in 1982 when the ozone layer was being removed, when data were released from the ozone column measured by the Japanese Syowa station (69H, 39E), located in Antarctica. Measurements began in 1964 and showed that since 1975 ozone had a clear downward trend.
In the rest of the stations scattered throughout Antarctica, ozone-like behavior was observed, and in all of them ozone decline began in the 1970s. In addition, scientists found that ozone decline began in the southern spring and disappeared at full speed. World scientists were overwhelmed because if the process went ahead they could have serious consequences for man and the planet.
Years later, J took an important step in understanding what was known as the ozone hole. Lovelock. In fact, the English scientist embarked on a project that aimed to study the dynamics that occurred in the high atmosphere. To do this, he focused on those substances emitted into the atmosphere very often that had a long life before their destruction. Thus, following in the footsteps of these substances, he discovered how they arrived in Antarctica from the places where they formed.
He called markers to those substances he was going to use to know the behavior of the atmosphere. After analyzing various substances, he selected the CFCs for this work, due to their use and stability. As he followed the CFC footprint, he realized they were in high concentration in Antarctica.
It was considered that the CFCs were very stable and that they did not affect anything, so many totally different industries were used for many purposes. But Molina and Rowland demonstrated in 1972 that in the CFCs ultraviolet radiation released chlorine that destroyed ozone.
When all this was known, environmental groups began campaigns against the CFCs to raise awareness among the people. In this way, the position against the CFCs was broadened. Politicians, faced with the seriousness of the situation, supported the prohibition of CFCs in several conferences, always slower than desired by environmentalists and faster than desired by the chemical industry.
It was first discussed at the Ozone Layer Conference in Stockholm (1972). In 1977 experts met in Washington to carry out the Global Ozone Layer Plan. Four years later, the United Nations proposed a treaty on the ozone layer problem. The first international agreement was signed in Vienna in 1985, where the intention to investigate the consequences of the lack of ozone layer was exposed.
But the best-known agreement is the one signed in 1987 in Montreal. It was signed by 165 countries, which brought together 90% of world production, and its goal was to end ozone destructive substances. They established two dates: 1996 for developed peoples and 2010 for other peoples. An agreement to meet at least every four years was reached to strengthen this agreement. The most important were Vienna (1995) and Montreal (1997). Currently 180 countries have signed the Montreal Convention.
Bibliography
- Science of Nature
Vol. 12, Planet (1997).
How Nature Works
Debate (1992).
Uriarte, A.
Ozone: The Catastrophe That Does Not Reach
Third Press (1995).
Cacho, J. and Sainz de Aja, J.
Antarctica: the ozone hole.
Tabapress (1989).