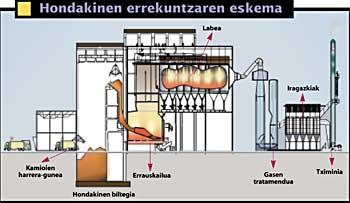
Where do dioxins occur?
2004/10/01 Roa Zubia, Guillermo - Elhuyar Zientzia Iturria: Elhuyar aldizkaria

Somewhat confusing. Apparently it is the only substance, such as ammonia or sulfuric acid, but dioxin is not the name of a single molecule. When you talk about dioxins you talk about a family of substances.
In addition, within this family there are two groups: polychlorinated dibenzo-p-dioxins and polychlorinated dibenzofuranas. Therefore, sometimes, instead of just dioxins, we talk about dioxins and furans. And through these names they represent a very extensive family. In total, 210 molecules are included in this group of dioxins, with very different sanitary effects. Some do not cause harm, others are very dangerous and worried products, especially because very small doses are enough to cause harm.

These molecules do not dissolve in water, so in living tissues accumulate in fats, that is, it takes about seven years to eliminate half the amount of body dioxin. Dioxins also accumulate in soil, plants, etc. However, the only source of dioxins are not incinerators, which must be taken into account in the matter.
Although there are many, dioxins are very difficult to detect in the atmosphere. On the one hand, the concentrations are very low and necessarily require precision methods that chemists must perform in clean chambers to avoid contamination of samples with other molecules.
Electron capture chromatography has normally been used, but it is a fairly complex technique, so many chemicals currently detect dioxins through highly accurate mass spectroscopy. On the other hand, these are highly toxic compounds, so the patterns used in the analyses are also toxic. Therefore, in addition to a difficult analysis, it requires many security measures.
Wherever you want

Man does not use dioxins at all, that is, they are not intentionally synthesized molecules, but side products of different processes. They are generated in any combustion of organic matter, in very small quantities but they are formed. In short, they are organic molecules that contain chlorine atoms, which means that molecules based on carbon atoms are formed whenever chlorine atoms are burned together.
This is what happens, among other things, in nature. When a forest is burned, much organic matter is consumed and chlorine atoms are always involved. The same occurs in volcanic eruptions, which always detect dioxins in the atmosphere in post-eruption analyses. There is no doubt that they are molecules that generate nature, so the reports related to dioxins always mention these two sources. However, the amounts generated by nature are not the same as those generated by man.
There are numerous sources of dioxins by human action. It is one of the main industrial sources, emitted by the cement, paper, metal, polymers and those who use wood and coal as fuel. It also emits waste incinerations and, outside the industry, diesel engines and domestic fires stand out

But in addition to all this, other combustion, such as tobacco itself, must be taken into account. The amount of dioxin generated by burning a cigar is not high, although it enters directly into a person's airway. From the health point of view, it is much more dangerous than that generated by the chimney of an industry, since the concentration of dioxins that the smoker introduces next to breathing is very high, much higher than the atmospheric concentration.
Analysis
Many sources, with the consequent risk of dioxins. However, what source emits more dioxins to the atmosphere? If pollutants are dangerous and have such an impact on health, what are the sources that concern us most?
They have carried out numerous studies. In the United States, for example, they invested a lot of money in the second half of the 1980s to answer this question. On behalf of the EPA (Environmental Protection Agency), numerous analyses were carried out, sources were identified and it was concluded that the main source of dioxins was incineration of waste. In addition, with any other source there was a big difference, with incineration responsible for 71% of harmful dioxins.
This research has long been carried out, and during that time incinerators have greatly improved. As a result, the proportion of dioxins generated by incineration has been significantly reduced. However, reports published by the EPA indicate that incineration remains the main source of dangerous dioxins.
Temperature issues



From a chemical point of view, there is a temperature range for the formation of dioxins. Normally, most dioxins occur when the combustion process occurs at 700 C. However, in most cases, the chemical medium consists of metals that act as catalysts.
Not all metals catalyze reaction, for example, have shown that aluminum does not facilitate reaction. Unfortunately, metals that catalyze reaction well are common in waste: when there is iron and especially copper, dioxins are formed at much lower temperatures. With copper 400 C are enough to produce dioxins.
Therefore, electrical cables contain the raw materials suitable for the production of dioxins: on the one hand, the insulation is a polymer containing chlorine, PVC, and on the other, the cable itself is a copper wire. This means that the risk of dioxins is in a very wide temperature range.
Therefore, it exceeds at least 850 C and in some cases 1.100 C. Dioxins are dissolved at these temperatures, but the problem does not end, the gases obtained must be cooled and when cooled dioxins are reformed.
Other factors
By burning organic matter it is very difficult to control well the temperature of combustion and the resulting products. The most advanced technology can also not prevent the generation of toxic compounds, so it is necessary to add other resources for its treatment. In addition, temperature is not the only factor to control.

The EPA has made great efforts to reduce dioxin emissions from incinerators. In fact, the experts of the organization proposed to control three factors of combustion: the temperature, the combustion time and the swirls that are generated in the process.
This means that with the modification of these three factors many experiments were carried out and the conditions that provided the best proportion of toxic and non-toxic dioxins were identified. According to reports published by the EPA, the dioxin spill has not been completed, much less, but the results have been satisfactory.
The problem will never be completely solved. But the goal is that at least the amount of hazardous substances is acceptable. And not only that, it is essential to know well the damage these substances can cause to health.
17 of 210 toxic molecules
Within a group of dioxins there are many substances, some toxic and others not.
They are actually two molecular families, polychlorinated dibenzo-p-dioxins and polychlorinated dibenzofurans (dioxins and furans). They are often expressed by PCDD and PCDF abbreviations.
In short, the difference between these two families is only an oxygen atom, but the fluctuation of the same atom causes many changes in the structures and characteristics of the molecules.
That is why they are not two molecules, but two families. Depending on the hydrogens and chlorine of each molecule, there are numerous possible combinations in the groups of dioxins 75 and 135 and furans respectively.
The basic structures of these families are:
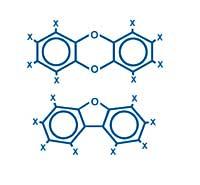
Where X is written there may be a hydrogen or chlorine atom.
In total there are 210 molecules, of which only 17 are toxic. For example, burning a cutlet produces many dioxins, but it is very difficult for one of these seventeen to occur in enough quantity to be dangerous.
Chemicals indicate by numbers where the chlorine atom is in each molecule. For example, the most known and toxic of the 210 is 2,3,7,8-PCDD:
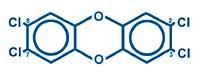
It has four chlorine atoms in positions 2, 3, 7 and 8. According to studies, it is essential that chlorine atoms are in those positions to be toxic. All toxins contain at least four chlorine atoms in those positions.
Toxicity measurement

The TEQ ( Toxic Equivalent Quantity) method is normally used to express the toxicity of dioxins. The idea is simple, rather than reporting the mass of dioxins, the result indicates the toxicity associated with that mass.
Not all dioxins are toxic and not all toxic ones have the same effect. After numerous studies, scientists have attributed these seventeen zero in one toxicity factors. The most toxic factor is 1 and the least toxic is 0.
Therefore, the TEQ method indicates the number of dioxins present, but the amount of each molecule corrected by the corresponding factor. Thus, ten molecules with a factor 0.1 and a single one with factor 1 will have the same toxicity.
Not only dioxins

Environmentalists campaign against processes and installations that emit dioxins and furans. However, these molecules are not the only products produced in combustion, nor are they the only ones that can have toxicity. The burning of organic matter generates and destroys thousands of compounds.
By burning the trash, in addition, many other compounds are generated. On the one hand, gases (hydrochloric acid, hydrochloric hydrofluoric, sulfur dioxide and nitrogen oxides) are generated, many of which produce acidity by joining the water.
On the other hand, simple aromatic organic compounds, polycyclic and organochlorine. Many of them are less toxic than dioxins, but occur at higher concentrations than dioxins.
Chlorine and aromatic rings Environmentalists will ask about the main polluting products and talk about organochlorines. Not only of them, they occupy an important place on the list. These include PCBs, dioxins and furans. They all contain chlorine atoms attached to aromatic rings. And that generates toxicity. There are other toxic substances with aromatic rings, but less toxic than organochlorines. In fact, the "contribution" of chlorine atoms hinders the degradation of the molecule by attracting electrons from the aromatic ring. In addition, in most cases the body removes substances through water (sweat, urine, etc. ), but PCBs, dioxins and furans do not dissolve in water, meaning they accumulate in the body. |

Gai honi buruzko eduki gehiago
Elhuyarrek garatutako teknologia