Structural dance of crystals

The word crystal is a technical term, iron or steel are crystals and glass are not. Specifically, the word crystal represents a material of crystalline structure.
Crystals, like any other material, undergo structural changes. For example, if the temperature rises a lot, they move from solid state to liquid.
But there are less noticeable changes, for example, in solid state. These are called solid-solid phase transitions and are produced by temperature or pressure changes. In addition, the electrical and magnetic properties of the crystal change frequently in these transitions, so they are very interesting for technology.
In Leioa they study the solid/solid phase changes of crystals. For this purpose, they have analyzed a group of crystals, the two double perovskites. However, before starting studies it is necessary to perform a kitchen work in the laboratory, perovskites should be synthesized.
Synthesis of crystals in laboratory
The synthesis of crystals is not an easy process. Mix and crush the powders of the ingredients and stabilize in the oven. For example, to cite a method of synthesis, to obtain perovskite Sr 2 NiWO 6 the powders of the compounds SrCO 3, NiO, and WO 3 must be mixed. The result of the mixture is also a powder, perovskite powder.
However, it does not always get the desired perovskite, so this glass powder should be characterized once synthesized. That is, in our example we must ensure that Sr 2 NiWO 6 is perovskite and not another; or the perovskite itself, but with certain impurities.
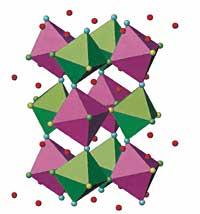
When perovskite has finally been obtained, different techniques are applied: X-ray diffraction, neutron diffraction, synchrotron radiation or Raman spectroscopy, etc. With all of them you get information of the structure of the crystal, calculating the location of the elements, their frequency of vibration, etc. To do this, the researchers have to travel to France and the United States, where there are no synchrotron or Raman pressure spectroscopy.
Versatile solid structure versatile
Therefore, the solid structure of the crystal can vary. But how? For example, if the pressure increases, the structure is tightened, and the glass atoms are closer to each other. This can cause an acceleration of the vibration frequency of the components. At any given moment the balance is broken and the arrangement of the components is modified, a solid/solid phase transition occurs.
Remember that many times, among other things, the electrical and/or magnetic characteristics of the crystal are modified, so at a certain temperature, as the conductivity of the crystal, can increase. In fact, Leioa is working on determining the pressures or temperatures to which these solid-solid transitions occur, as has already been indicated, with perovskites.
- Project title Characterization of phase transitions and phases in double perovskita.
- Objective Analysis of phase transitions and characterization of phases that occur in the families of several double perovskites that are oxides.
- Director Josu M. Igartua Aldamiz.
- UPV working team: J.M. Igartua, M. Insausti. University of Michigan: M. M. Gateshki. Florida International University: B. B. Manoun.
- Department of Applied Physics II.
- Faculty of Sciences and Technology.
Buletina
Bidali zure helbide elektronikoa eta jaso asteroko buletina zure sarrera-ontzian








