CLICK: Sound of lego molecules
2010/03/01 Aizpurua, Jesus Mari - EHUko Kimika Organikoko katedraduna Iturria: Elhuyar aldizkaria
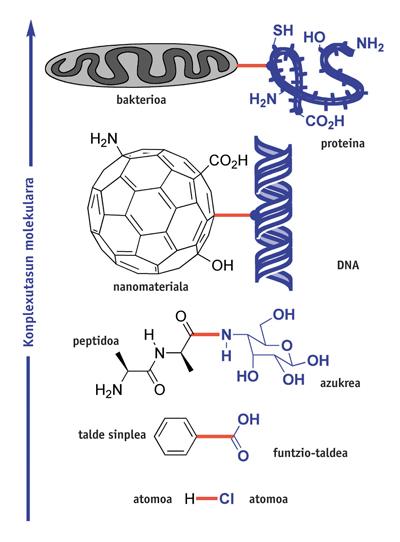
Chemistry is the science of union. Hundreds of reactions have been found to form chemical bonds. and XX. For centuries, and from them, increasingly complex molecules have been synthesized. But this activity has its limitations: those imposed by the molecular structure to be prepared. What is the current complexity limit of chemical synthesis?
Organic compounds containing diatomic molecules (HCl) or simple functional groups (C 6 H 5 CO 2 H) are easily prepared by giving one or more stages of synthesis. However, the preparation of polyfuntzional molecules with groups such as NH 2, OH, CO 2 H, requires many synthesis steps. For example, the preparation of glycopeptide binding the peptide and the image sugar requires more than ten steps of synthesis. Some of them are also intended to protect and unprotect NH 2 and OH groups, and cannot be used in large fragile molecules. However, each year more than 500,000 new molecules are synthesized using this type of classical synthesis by protective groups.
However, scientists aim to establish connections between increasingly complex molecular structures and perform them very selectively, for example between fullerene and a certain DNA nucleotide, or between bacteria and some special amino acids of a protein.
M. 2002 Danish melodies and K. B. Americans Sharpless claimed separately the discovery of the first click reaction. They observed that the reaction between alkine and azid accelerates 10 8 times with the addition of some copper catalyst (I). A finding of this type would not be noteworthy if this process had no other characteristics, very special: a) the reaction is almost always quantitative or of very good performance, b) it occurs at room temperature, c) it works surrounded by air in the water and biological means, d) it has a high tolerance to many functional chemical groups (it is a chemoselective) and e) the triazoles produced in the reaction are unstable, but not very toxic.
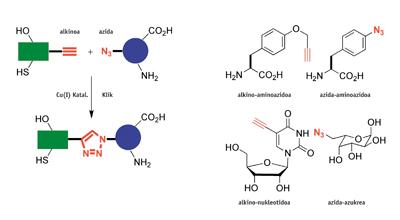
Chemistry turns click into a modular chemical synthesis. As with the Lego toy, complex molecules can be easily constructed by "clicking" other simpler ones. In addition, click reactions are simple to make scientists inexperienced in organic chemistry. Therefore, they are ideal to adapt to the synthesis of robots.
Since compounds containing alkine and acidic chemical groups are foreign to nature, click reactions are bioorthogonal, i.e. they do not require protective groups to provide bioconjugate or hybrid structures. Therefore, amino acids, sugars or nucleic acids that contain alkine and acidic groups can be used to make proteins, saccharides or "clickable" DNA. All these characteristics have allowed the applications of click reactions to have quickly exceeded the scope of organic synthesis and have been extended to biochemistry, pharmaceutical chemistry, clinical diagnosis, material science and nanoscience, among others. Over 12,000 articles related to click chemistry have been published in scientific journals in the last seven years.
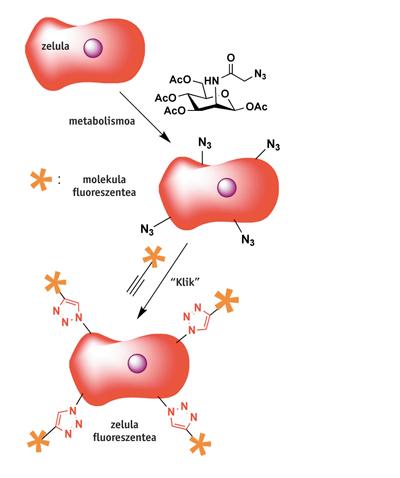
Chemistry has had many striking uses, so it is impossible to report all of them here. We will see a couple of examples: the first is the C of the University of Berkley. R. It is the most fluorescent living cell obtained by Bertozzis. Once the cells are fed with a special acid sugar (mannose azide), the membranes are coated with a group of whips. Immediately after the click reaction with the fluorescent molecule containing the alkine group, the cells become fluorescent… and survive! Similar to this procedure have also been tested on larger living beings such as zebrafish.
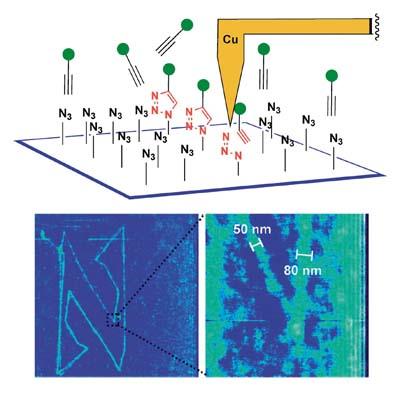
Clicks have also reached nanotechnology. In 2009, J. F. The American Stoddart got a chemical nanomarrization on the silicon plate. For this purpose he used an atomic force microscope (AFM), provided with copper nanopunts, on a silicon sheet coated with groups of azids. After impregnating this sheet of 4-pentine acid alkaline and alcohol, write on it the letter N with nanopunta. Since the reaction is only catalyzed in uneven places, once the iron is cleaned with alcohol, the nanometric image of the letter N is associated with the surface. Logically, this technique allows almost any nanolithographic biomolecule on surfaces of different materials.
It is therefore difficult to predict what new structures scientists will build in the near future, but one thing is certain: click sound will be heard more and more in laboratories around the world.

Gai honi buruzko eduki gehiago
Elhuyarrek garatutako teknologia





