Through heterogeneous catalysis in search of sustainability
XIX. and XX. The industrial revolution of the nineteenth century brought with it not only new products but also changes in the manufacturing processes of them. Iron and steel are the most powerful compounds they took in this revolution; however, oil is the source that has allowed to transform the widest range of products possible. Most of the oil, refined, such as gasoline, diesel and kerosene, is destined to the energy market, while much is destined to the manufacture of lubricants and asphalt. The production of plastics is another of the revolutions related to oil. The main properties of plastics are adaptability and chemical resistance, especially taking into account their low weight. But what factors or circumstances make crude to become so many products?
The importance of catalysis
Undoubtedly, the nature of a product depends on its antecedents, that is, on its molecular structure. One of the clearest examples is polymers, in which the same monomers united by rows can form a polymer structure of a certain length. Basically, the chemical structure is the same, but as discussed above, when it becomes a long chain, these polymers acquire additional properties. In addition to reaction conditions (temperature, pressure, flows, etc. ). The nature of the catalyst is usually one of the factors that determines the viability of a product. The term “catalisi” was first used by researcher Berzelius in 1836. However, catalysis was also used in ancient fermentation processes, such as wine production. Despite the fact that users at the time had no knowledge of this concept, the compound that helped speed up fermentation and obtain a certain product was a biocatalizer.
Catalysts can be classified according to their physical and chemical nature. Biocatalizers are catalysts of biological origin (enzymes in general). They are also used industrially for the production of food (bread, Saccharomyces cerevisae) or fuels (bioethanol from sugar cane in Brazil). The complexity of the enzymes allows to lead more difficult reactions than in many chemical processes. However, taking into account the demand for consumption in Western countries, the main drawback is the low production speed or the disappearance of active ingredients, especially in comparison with the thermochemical processes.
In order to make the reaction speed as fast as possible, in recent decades, many mineral catalysts such as sulfuric acid or sodium hydroxide have been developed. Finally, by changing the chemical composition of the catalysts, in addition to accelerating the formation of the different products, you can get the process to go to a certain product at an affordable price. One of the clearest examples of chemical catalysis is the combustion of the smoke of cars. In fact, in the composition of the smoke of the cars various compounds of hydrocarbons and nitrogenates are generated. To reduce their degree of contamination, chemical catalysts formed by different metals are usually used, in most cases in solid form.
In the industry, on the other hand, a homogeneous catalysis is used to make most of the compounds. The contact between two liquids or two gases allows a homogeneous catalysis between two equal phases. Of course, the main drawbacks, in addition to having a low renewables, are the problems derived from the process. The oxidation of the installation and the separation of the product/catalyst provoke an increase in the costs of the entire system and, in particular, the generation of many toxic currents so polluting for the environment.
XXI. The revolution of dependent sustainability: biomass and heterogeneous catalysis
The sustainable economy and lifestyle focus on the source of current products and their process of elaboration. However, our production systems are mainly based on fossil sources: oil, natural gas or coal. Therefore, this system totally hinders the sustainable implementation of these indicators. Given this lack, a study has been carried out that combines biomass with heterogeneous catalysis as an alternative: On the one hand, a renewable source and, on the other, a suitable way to reduce the toxicity of many currents.
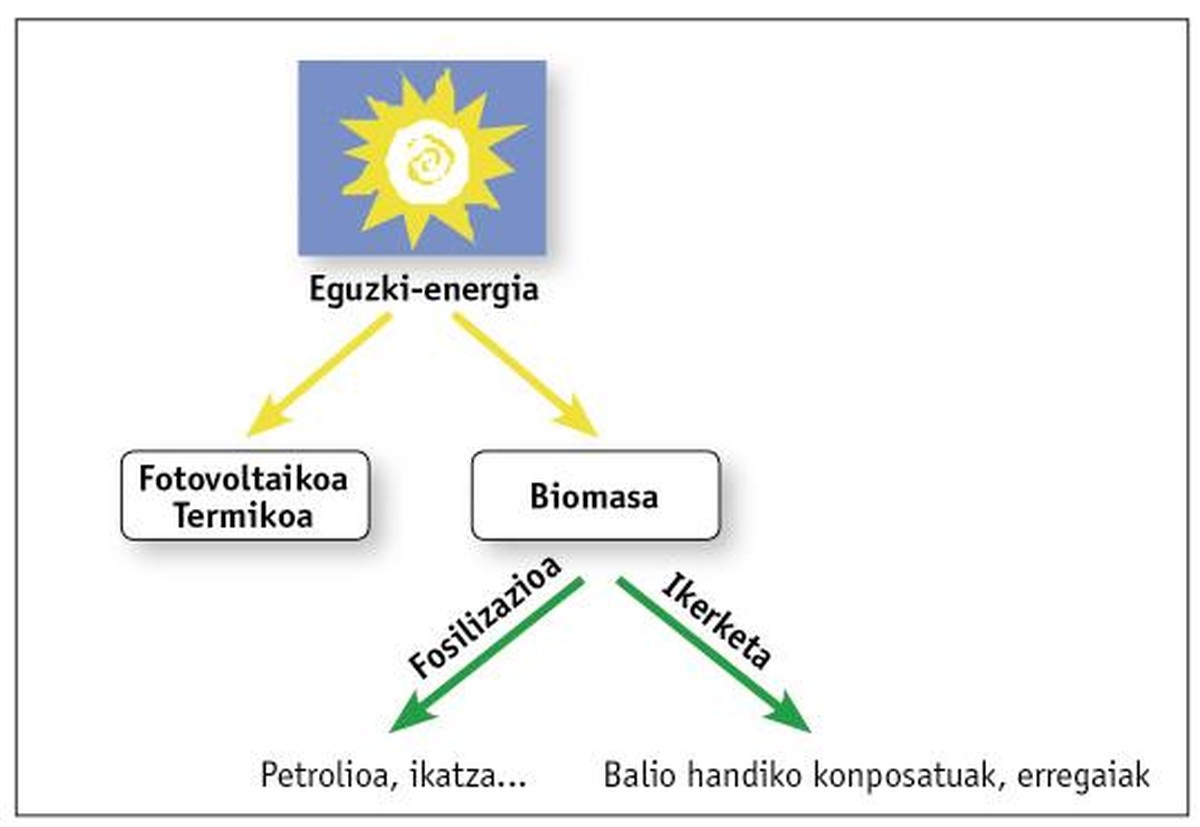
Oil is the main current source and the aim of this work is to open new paths to biomass that generates the land itself. As for definition, biomass is concentrated solar energy. After complex enzymatic processes, it stores this energy in the form of carbon. Fossilization has led to the formation of oil and some compounds over millions of years (see figure 1). The aim of this research is to use “young” biomass.
Experts have identified 30 main compounds from biomass. Among them is the fural, axis of this study. Furfural can now be obtained through an industrially developed process and is used in the manufacture of various resins, pharmaceuticals and lubricants. China is the main producer. In this country, the lack of regulation causes enormous environmental damage. The main source of problems is the use of homogeneous catalysis, in this case sulfuric acid or phosphoric acid. The study focuses on the synthesis of heterogeneous catalysis for the conversion of biomass into furfurrow.
The fact that a material is heterogeneous means that the main phase in which the reaction occurs and the catalyst are found in different states, that is, the reaction in liquid/gaseous phase and the catalyst in solid phase (Figure 2). In these cases, the reaction occurs only in contact with the catalyst. Heterogeneous catalysts can be classified, in most cases, according to their properties: modifying the physical properties of all the material (in most cases, the size of the pores) and changing the nature of the active centers of the layer.
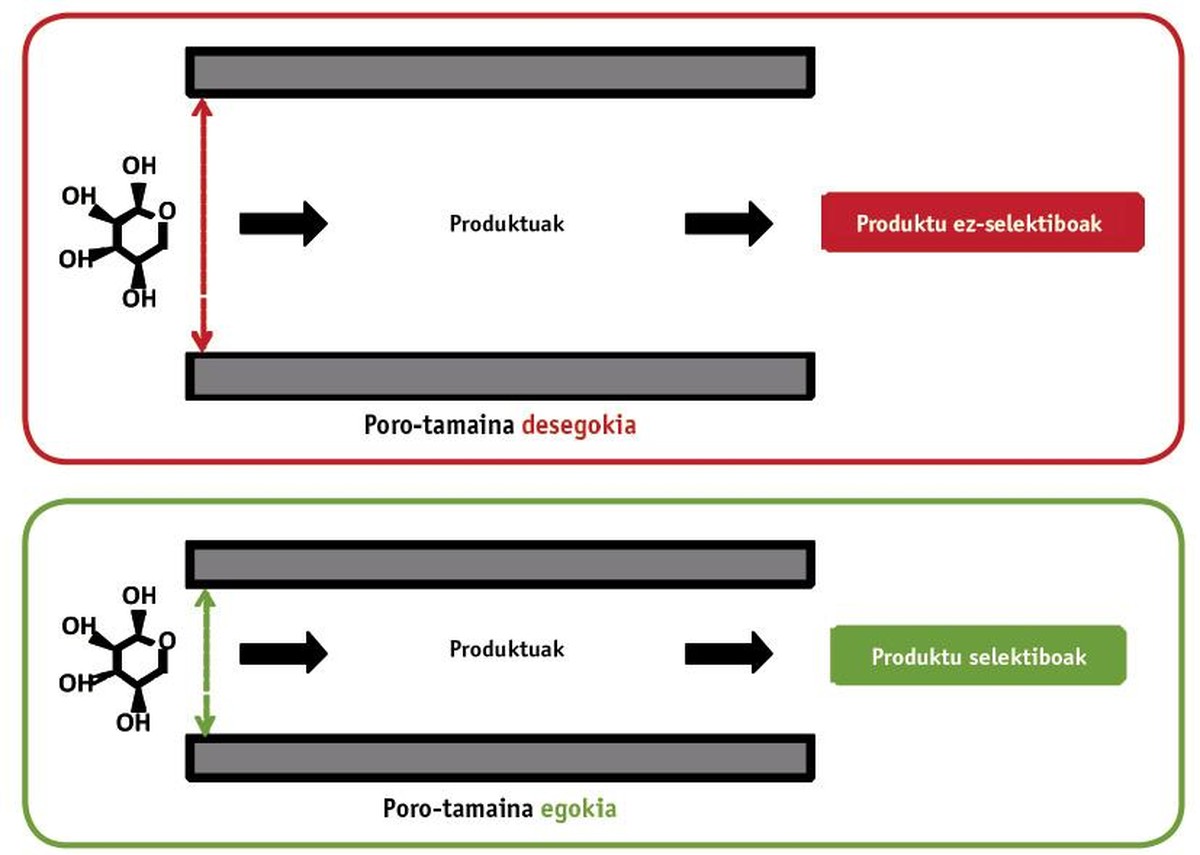
The essence of heterogeneous catalysis is to classify the molecules according to their size. For this purpose, large layer materials are needed. This is achieved using porous materials (sponge type). Take as an example the fural: in this case we leave the sugar called xylose, which suffers a dehydration reaction to turn it into furrow. Of course, for the reaction to occur, this sugar must reach the conversion point. For this it has been necessary to design a suitable porous structure, taking into account that the xylose has a size of 0.72 nm. If the pores are too large they can form unwanted products. On the other hand, the optimization of the pore size significantly facilitates the production of furfurrow (see second part of figure 3). The results show that the variation of the material synthesis temperature in 10 °C produces decisive changes in physical properties. One of the main results is the detection of the most suitable porous size of an acid catalyst formed by silicon.
In order to periodically modify a single parameter, the second part of the study has addressed the modification of the chemical properties of materials composed of magnesium and fluorine. Based on materials with very similar physical properties, a synthesis procedure has been developed to modify the quantity and nature of chemical centers. In this case, different ways have been found to obtain furfal. The strong acid centers allow the direct path, while the weak define the path of reaction that is given through the formation of different intermediate compounds. Logically, both cases have given different product yields, and above all, centers have been identified that have accelerated the speed of reaction. The quantity of both centers has varied between 0 and 100% and the results have allowed to synthesize a catalyst containing an optimal quantity.
One of the advantages of these heterogeneous catalysts is that they facilitate separation after reaction. Once these reactions have been carried out in liquid phase, it has been possible to filter and recover the solid catalyst. In addition, to evaluate the viability of this process, the catalyst has been reused, obtaining the same performance values obtained previously. In this way it has been possible to avoid problems of separation with homogeneous catalysis and to design a more “green” process.
As the work has concluded, the catalysis and technology developed can mean the evaluation of many processes that today are not viable and achieve a higher level of sustainability, in addition to offering the possibility to continue taking steps that the environment will thank us enormously.
References References References References
Buletina
Bidali zure helbide elektronikoa eta jaso asteroko buletina zure sarrera-ontzian