Graphine competing with graphene
2012/05/01 Elhuyar Zientzia Iturria: Elhuyar aldizkaria
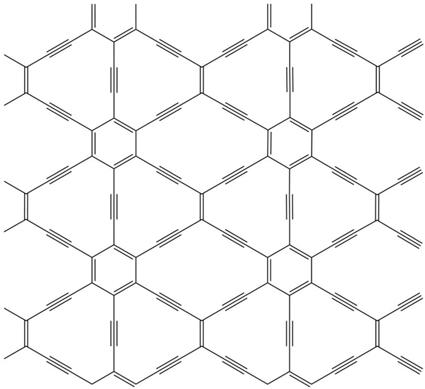
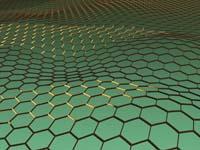
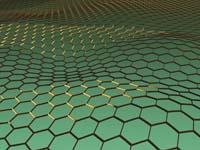
Graphene is a material with a monoatom layer thickness of carbon whose mechanical and electronic properties have made it a material of great hope. The graph is very similar, but its carbon atoms are not ordered to form hexagons. Between the carbon atoms of graphine double and triple bonds are formed, so it does not always adopt a hexagonal symmetry.
In fact, the graphs form a complete family of symmetry compounds, and one of them, the 6,6,12-graphine, suggests in the journal Physical Review Letters that graphene, its electronic properties could be even more interesting. The rectangular symmetry of the material would allow to arbitrarily control and limit the conductivity direction, unlike graphene.
At the moment, these properties have been announced by computer simulation and there is no experimental evidence, only one graph has been synthesized to date and is another. Confirming the prognosis, the electronic organization called Dirac cone would not be limited to hexagonal symmetry. The Dirac cone is a concrete way to organize the energy levels of electrons, allowing graphene to have such fascinating electronic properties. Physicists believed that Dirac cones were limited to hexagonal symmetry.

Gai honi buruzko eduki gehiago
Elhuyarrek garatutako teknologia