Dynamics of atmospheric reactions
2003/07/01 Andonegi Beristain, Garazi - Elhuyar Zientziaren Komunikazioa Iturria: Elhuyar aldizkaria
The atmosphere protects us from the Sun and this protection is due, among other things, to the ozone layer. The ozone layer is 19-48 km from the Earth in the stratosphere and is due to its high ozone concentration.
Ozone is a molecule made up of three oxygen atoms that form in the stratosphere itself. In recent times, however, ozone levels in the stratosphere are declining markedly due to the molecules generated by human action. In fact, the rate of ozone extinction has accelerated and therefore more ozone disappears than is generated. Therefore, they aim to clarify the details of the reactions between ozone and these molecules.
The dynamics of chemical reactions are being investigated precisely in the Department of Physical Chemistry of the Faculty of Pharmacy of Vitoria-Gasteiz. Researchers have studied the reaction between OH radical and hydrogen chloride (HCl), among others. This reaction produces water (H2O) and chlorine atoms (Cl), chlorine being one of the most important agents for the elimination of ozone.
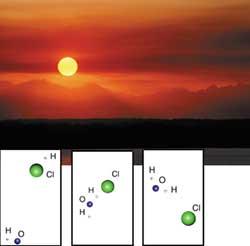
On the other hand, the reaction between chlorine atoms (Cl) and methane (CH4) has been investigated, a reaction that neutralizes chlorine atoms and thus protects ozone.
Molecular dynamics of chemical reactions
The speed of reactions in the atmosphere must be released according to the forces. In fact, in the atmosphere there is no thermal equilibrium and kinetics cannot be applied to investigate the speed of reactions, such as the rate of ozone extinction. These reactions must be analyzed through molecular dynamics, that is, released according to the shock energy of molecules, spin energy and vibration energy.
In dynamic analysis, first of all, all these energies are represented in a mathematical function: the potential energy surface, PES (Potential Energy Surface). This mathematical function depends on the potential energy and position of atoms.
Then, once this potential energy surface is known, researchers simulate intermolecular shocks. This selects initial energy, orientation and speed for molecules. The Newton equation of forces is then applied and the reaction speed, the energy state of the products, the vibration state of the molecules, etc. are released from the simulations.
These simulations must be carried out in conditions of millions of principle and, moreover, it must be taken into account that, depending on these initial conditions, in some cases the atoms will react and in others not.
They use a virtual supercomputer at the Faculty of Vitoria. The supercomputer is made up of hundreds of campus computers working together to release the variables they investigate during the night.
The results that will finally be obtained are fundamental in the chemistry of combustion, astrophysics, interestial studies and spatial research, and, although they seem simple reactions, they have a great importance, for example, in the formation and extinction of ozone.
- Project title Electronic,
dynamic and kinetic structure of polyatomic reactions. - Objective To
develop computational theoretical methods for the research of polyatomic bimolecular reactions involved in atmospheric processes. - Principal Investigator Ernesto
García Para. - Research Group M.
Martínez, C. Sánchez, A. Saracibar. - DepartamentQuímica
Física. - • Pharmacy
(Faculty of Environmental Sciences) - •

Gai honi buruzko eduki gehiago
Elhuyarrek garatutako teknologia




