An electron in fraganti

In a grain of sugar there are millions and millions of atoms. (Photo: JPPI/Morguefile)
Of course, electrons are so small that they cannot be seen at first glance. Take a grain of sugar. It is very small, if we leave on the table and move away a few meters, we can not see it. Suppose we widen this grain of sugar until it is the size of the Earth. Then we would see very big. The molecules that form the grain would have the size of a house. And the atoms of the molecules would have the size of the rooms. Atoms have a nucleus (where protons and neutrons are concentrated). The size of this core would be equal to a specimen of dust located in the center of the room. They form the nucleus between all protons and neutrons, as they are very small. Because electrons are much smaller. The mass of an electron is 1,800 times smaller than that of a proton.
For atoms, far from being the size of a room in a house, are ten million a millimeter. Considering this and the comparison above, imagine how small an electron is!
Say and do

Doesn't it seem impossible to record a single free electron? Well, the researchers who have achieved it have said that it was a fairly simple task when they raised how to do it. The biggest challenge to overcome was to make electrons visible to the naked eye.
They got it with several steps. The basic principle was the repulsion force of electrons. Electrons repel the atoms around them, as they all have electrons on the surface layer and repel the two particles with the same load. By repelling they form a small hole around it, a bubble or. By placing a free electron in a normal liquid, it would cause a great pressure on the electron hole and destroy the bubble.
Scientists wanted the opposite, that is, not to lose the gap generated by the electron to make it more evident. To do this, they released an electron from its original atom and placed it in a special liquid: liquid helios. Helium, under normal conditions, is a gas and to take it to liquid state it is necessary to cool it a lot (up to about -269ºC). At such low temperature, helium exerts very low pressure. Under these conditions, the bubble formed by the electron in its vicinity was not eliminated and became more evident. However, it was still too small to see it at a glance.
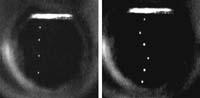
Through a specially designed experiment they have managed to photograph the electron. (Photo: H. Maris and W.Guo)
To further increase the bubble they used the sound. As the sound waves moved into helium, pressure waves occurred in the bubble. That is, when a wave hit the electron, the pressure around the electron increased and the bubble decreased, but when it moved away from the electron the pressure decreased greatly and the electron bubble became much greater, and then it was visible.
In the moments when the bubble became visible the assembly was lit with a special light and everything was recorded with a domestic camcorder. It's not a little, isn't it? The scientists themselves were also surprised to see what they did.
Published in 7K.
Buletina
Bidali zure helbide elektronikoa eta jaso asteroko buletina zure sarrera-ontzian