When Einstein collided with light

In 1905, Einstein explained the photoelectric effect. For this he had to rely on the theory of the many. To analyze the history of quantum discovery, first look back. It is necessary to analyze the debate on the nature of light.
In 1801 the English physicist Thomas Young wanted to better understand what light is. Until then, Newton said that light was made up of small particles. But Young passed the light through two slits and, like the floating waves, saw that it was diffracting. Therefore, I deduced that light is the wave. Einstein gave the reason to both when he explained the photoelectric effect.
Photoelectric effect
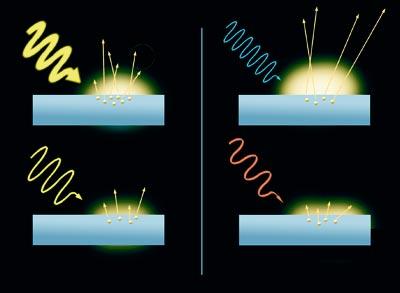
It is a curious effect. When light rays impact the surface of a metal, they cause electrons to be extracted. This is the photoelectric effect. In addition, the result varies depending on the color of the rays. Electrons released by blue light move faster than those released by red light. In fact, the higher the energy of the light beam (that of blue light is greater than that of red light), the faster the movement of electrons. On the other hand, the number of electrons released from metal depends on the intensity of light, the greater the intensity of light, the greater number of electrons. However, light does not release electrons without having minimal energy.
He started from the theory of Einstein Planck. This theory takes into account that light is the wave, that it has a frequency and that frequency is proportional to energy.
The wave is a simple example. Frequency is the time interval between one wave and the next; the higher the interval, the lower the frequency. Coming back to light, red light has less frequency than blue, so, according to Planck's theory, energy is much lower. In fact, strongly united electrons cannot be released by low-frequency radiations. They require a lot of energy.

Thus, in 1905 he gave a detailed explanation of the photoelectric effect. Assuming that the electrons that are released have certain energy levels, like Planck, the young Albert Einstein proposed that with radiation the same happens. Einstein realized that both energies are united in the photoelectric effect, so the shock must be interpreted as a clash between two particles, that is, that light acts as a billiard ball. He called the light particle a photon and claimed that the energy of the photon is proportional to the frequency of light, as in the case of the wave. Therefore, according to Einstein's hypothesis, light is both particles and waves.
This is the explanation why Einstein was awarded the 1921 Nobel Prize in Physics and not for the theory of relativity, although undoubtedly that theory and the rest of its contributions to physics were important. They probably win the Nobel Prize.

The winning work of the Nobel Prize in Physics is entitled: Über einen die Erzeugung und Verwandlung des Lichts betreffen heuristischen Gesichtspunkt. Besides giving an explanation of the photoelectric effect, it is a basic work for quantum physics, very revolutionary, according to the author.
However, although Einstein had great importance in the beginnings of quantum theory, the real revolution of quantum was XX. It began in the second quarter of the century and Einstein did not.
Einstein said that light can not only act as a wave, but as a particle, while Louis de Broglie said particles can also act as waves. But what did that mean? That any matter has an undulating nature. Yes, you can play as waves your chairs, dogs, trees or yourself. Logically, when it comes to very large bodies, the undulatory nature has no force, so this effect is not noticeable in the matter we see, but it does at the atomic and lower level. That is, if a beam of electrons impersonates a wall with two slots, such as the waves of the sea (and not like the billiard balls) will interfere with each other, that is, they will behave in the form of waves. Proof of this are some of the most widespread techniques today such as the electronic microscope.

particles and waves.
From that moment on we cannot distribute the concept of particle and wave. No. It can be said that they are two perspectives of the same concept. In some cases matter appears in the form of particles or corpuscles and in others in the form of waves. It took years until the physicists accepted those conclusions.
This work was the basis of the new branch of physics developed thanks to the theory of quantum physics. Einstein's contribution is taken as the real starting point of quantum physics, as Planck only contributed the concept of how much. Subsequently, from the work of Planck and Einstein, many other physicists gave a surprising interpretation to quantum physics.
Jump to atoms
Among those who gave a new course to quantum theory, Niels Bohr highlights. The Danish physicist applied to atoms the theory that applied to radiation. That is, he cultivated the atomic model. In his opinion, the electrons of atoms could not have any energy. Around the core they moved in several spots, each with a certain energy level. The closer the electron is to the nucleus, the more energy is needed to evacuate it.
Bohr's atomic model is planetary, that is, electrons orbit around the nucleus. According to Bohr, electronic orbits are stable and occur at certain energy levels, that is, their energies are quantified.

For example, when hydrogen is heated in a closed, transparent container, it emits light called the hydrogen emission spectrum. The Bohr model explains very well why the light emitted is of certain colors or frequencies, and not white (if all the light colors were emitted would be white). All this is related to the discrete or quantized energy levels of electrons.
However, the Bohr model can only explain the spectrum of hydrogen. Although suitable for the hydrogen atom, he was not able to explain the atomic spectrum of systems composed of two electrons. In fact, the Bohr model only showed single-electron atoms. And yet it's not a little, don't think about it! On the contrary, thanks to Bohr the doors were opened and the rest of physicists could analyze more complicated atoms.
The quantum revolution took 15 more years to appear the atomic spectra of the multi-electron elements. This also meant the disappearance of the planetary atomic model.
In 1926, Schrödinger published the famous equation that bears his name. The planetary atomic model was overcome, as this equation shows that electrons behave in waveform in atoms. Therefore, one cannot speak of electron orbits, since a wave is not found in a given physical place, but in a wider area. And this is not called orbit, but orbital. That is, the orbital gives us the probability of finding electrons in one place. Therefore, instead of talking about electron orbits, we have to talk about the probability of finding electrons.
Without a doubt, all these theories are the result of the work done by Planck and Einstein. Theoretical physics took great steps, but it did not remain in a mere theory. It had many applications. These apps would also surprise Einstein.
Fission, daughter of relativity
Footprint in novelsAt present it seems that the Nobel Prizes in Physics are more distributed, so to speak. In fact, in recent years works from different areas of physics have been awarded, such as superconductors, cosmic neutrinos, condensed Bose-Einstein, etc. They are all very different subjects. In the past, with the passing of the year, the subjects related to the photoelectric effect or the nature of the atom were the ones that received the most prizes. It all began in 1918 with Planck. In 1921 Einstein received the Nobel Prize in Physics for explaining the photoelectric effect. However, the photoelectric effect and quantum physics were not intact. The physicists continued to work with good results. The following year, in 1922, Bohr received the Nobel Prize in Physics. Einstein applied the theory applied to light to atoms, that is, he proposed the atomic model. In 1923, Millikan received, among other things, the prize for confirming the two previous theories. In 1925 he was picked up by Franck and Hertz for studying the clashes between electrons and atoms. It was donated to Broglie in 1929 for discovering the undulating nature of electrons. According to this, in addition to light, any matter can act as a wave. In 1932, through quantum mechanics, Heisenberg was also awarded. In 1933, Schrödinger and Dirac were awarded for developing the atomic model proposed by Bohr. The first, for example, wrote the mathematical equation that gave waveform or information of any particle. As can be seen, the footprint of the photoelectric effect in the Nobel Prize in Physics is not small. It is significant. For several years, work related directly or indirectly to this subject has been awarded. |
Buletina
Bidali zure helbide elektronikoa eta jaso asteroko buletina zure sarrera-ontzian








