“Today you have a special flash”
2018/11/15 Irene Urcelay Olabarria - Irakasle atxikia Fisika Aplikatua II Sailean/EHU | Raquel Fuente Dacal - Irakasle atxikia Matematika Aplikatua Sailean/EHU | Iñigo González de Arrieta Martínez - Doktoregaia Fisika Aplikatua II Sailean/EHU | Telmo Echániz Ariceta - Irakasle atxikia Matematika Aplikatua Sailean/EHU Iturria: Elhuyar aldizkaria
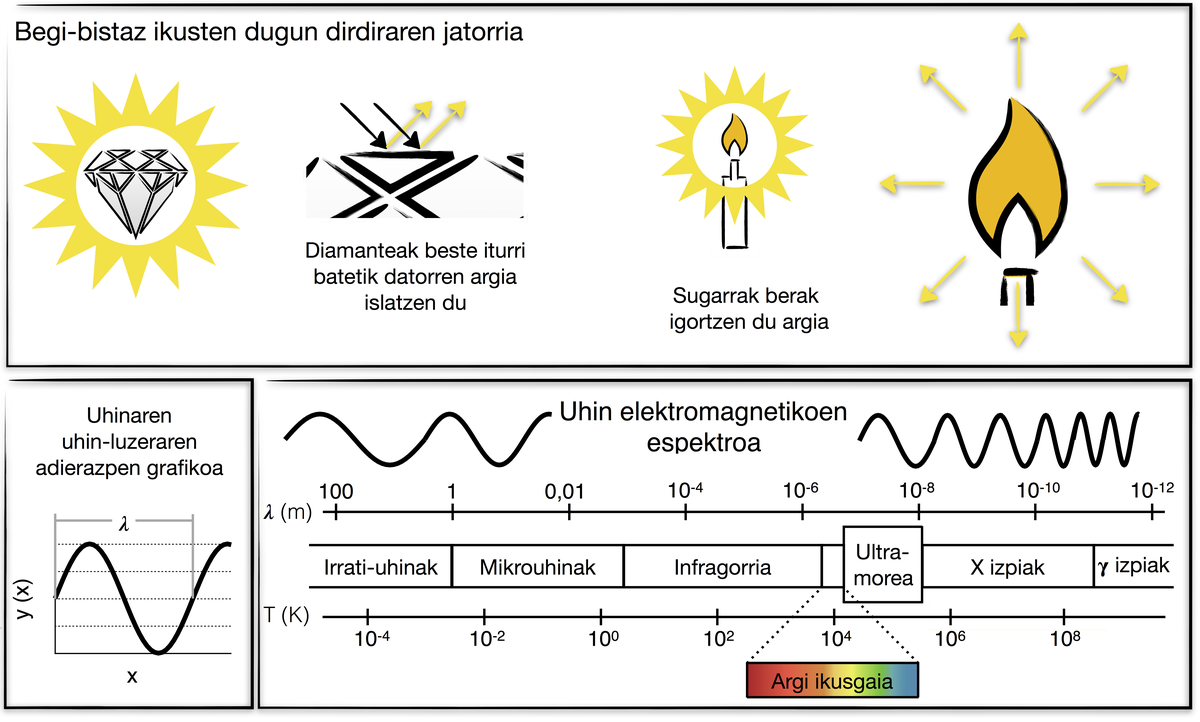
The origins of flicker can be light emission or light reflection. Let's analyze the brightness and that of a diamond flame of a candle that we see at first glance. If a lit candle is inserted into a dark room, its flame flashes, that is, the flame emits light. In the same room, in the dark, if a diamond is inserted, however, it does not look, it does not emit light. Therefore, when we see that the diamond flashes, we only see the light it reflects.
The light itself is the wave, the electromagnetic wave. Depending on the wavelength, there are different types of electromagnetic waves: radio waves, microwave, infrared, visible light, ultraviolet, x-rays and rays{. Until 1800 it was thought that the Sun only emitted visible light. Then F. W. Scientist and astronomer Herschel analyzed light from the Sun with a simple mercury thermometer and discovered infrared rays, using a prism that separated light into colors and analyzed the temperature of each color. Herschel was surprised to see that the temperature of the thermometer increased under red rays in a colorless area. These rays were called infrared rays. The Sun emits infrared rays, visible light and ultraviolet rays.
The human eye is only sensitive to visible light, i.e. to a very small part of the electromagnetic spectrum. Therefore, specifically, the diamond of the above example does not emit visible light in the dark. But does it emit another type of light?
Every body emits beams of electromagnetic waves. The diamond emits electromagnetic rays in that dark room (but not visible light). Hollywood stars, eyes of lovers and anyone else. The intensity of the light emitted by the bodies depends on the temperature of that body. In general, the higher the temperature, the lower the wavelength emitted by things. Bodies at room temperature emit waves from the infrared part of the spectrum, so the human eye cannot directly see that light. On the other hand, if the bodies are heated, sometimes they turn red as the radiation they emit becomes visible. Because of its relation to temperature, this type of radiation is called thermal radiation. Humans have used this property without knowing the physical bases for different applications. For example, glass craftsmen heat the glass and know whether or not the glass is at the right temperature to be made according to the intensity and color of the light it emits. The same could be said of the blacksmiths. Among animals there are snakes sensitive to infrared rays, which allows them to detect prey in moments of low or no light. If the human eye were sensitive to infrared light, we would see it in the darkness we know.
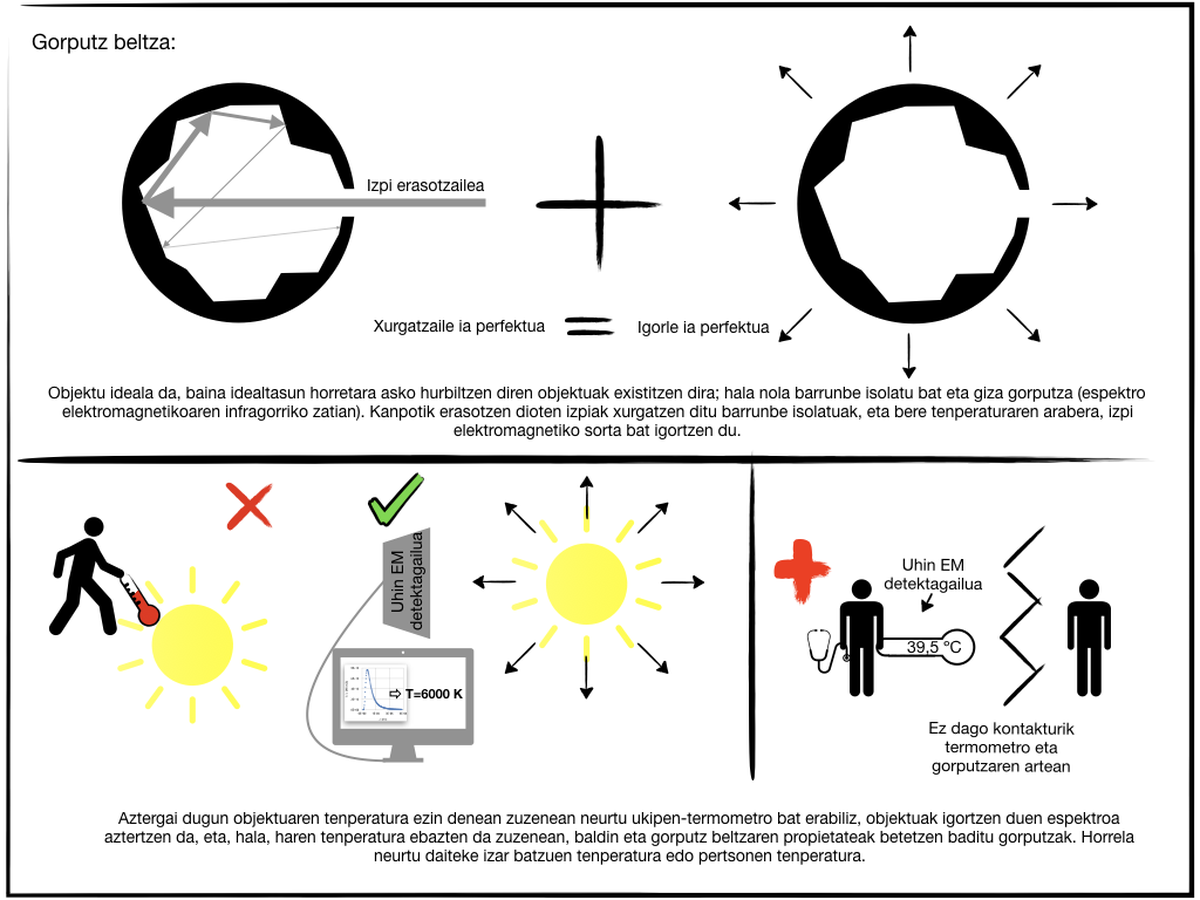
There is a special type of body: black body. It is a theoretical or ideal object that is characterized by being a perfect absorbent that allows the passage to the inside of all the radiation that affects the surface, that is, it reflects nothing, besides absorbing all the radiation that passes to the volume. Thus, it transmits nothing. In addition, thermodynamics demonstrates that the perfect energy absorbent is also the perfect emitter. The black body emits more radiation than any other body at the same temperature. Being a theoretical object, real approximations can be made using, for example, an isolated cavity. Radiation from a small hole in an isolated chamber is analyzed. The radiation entering through this hole remains in it, the system absorbs it and the emitted light depends on the temperature of the cavity. Another singular object that can be considered as a black body is human skin. The bark, which is at 33ºC, emits mainly infrared rays and, in addition, emits more radiation of wavelength of 5-20 ?m than any other body that is at that temperature, that is, the wavelength is practically the perfect emitter in this range. Therefore, in infrared ray cameras that are used for night or dark vision, the greatest intensity of flashes are those emitted by people, which allows their correct identification.
The maximum radiation intensity emitted by the black body is related to its temperature. Therefore, the detection of radiation emitted by the black body allows to directly calculate the body temperature. This property is used to determine the temperature of stars or the temperature of people without having to touch, for example. But is it possible to know the temperature of bodies that are not black bodies by analyzing the radiation they emit? Yes, let's see how.
The radiometry technique analyzes the energy emitted by the bodies and transmitted by electromagnetic waves. When measuring in the laboratory, the body we want to study is placed at the appropriate temperature and the radiation emitted is received. It also collects a series of electromagnetic waves emitted by a black body in the same state and is used as a reference. This calculates the emissivity of the bodies at a certain temperature,>. Once the emissivity of the body at different temperatures is known, the relationship between radiation and body temperature is direct, so that, analyzing the radiation emitted by the body in all conditions, the temperature at which it is located can be quickly known. This is very useful when it is not possible to measure the temperature of a material using a contact thermometer. For example, wear, friction and specific heat of the material are very important in cutting machines, and these quantities depend on temperature. Unfortunately, when the machine is working, the temperature cannot be measured by contact, so analyzing the radiation emitted by the material and its emissivity can be known at all times the actual temperature of the material. This is the technique developed in recent years for the study of body temperature: pyrometry.
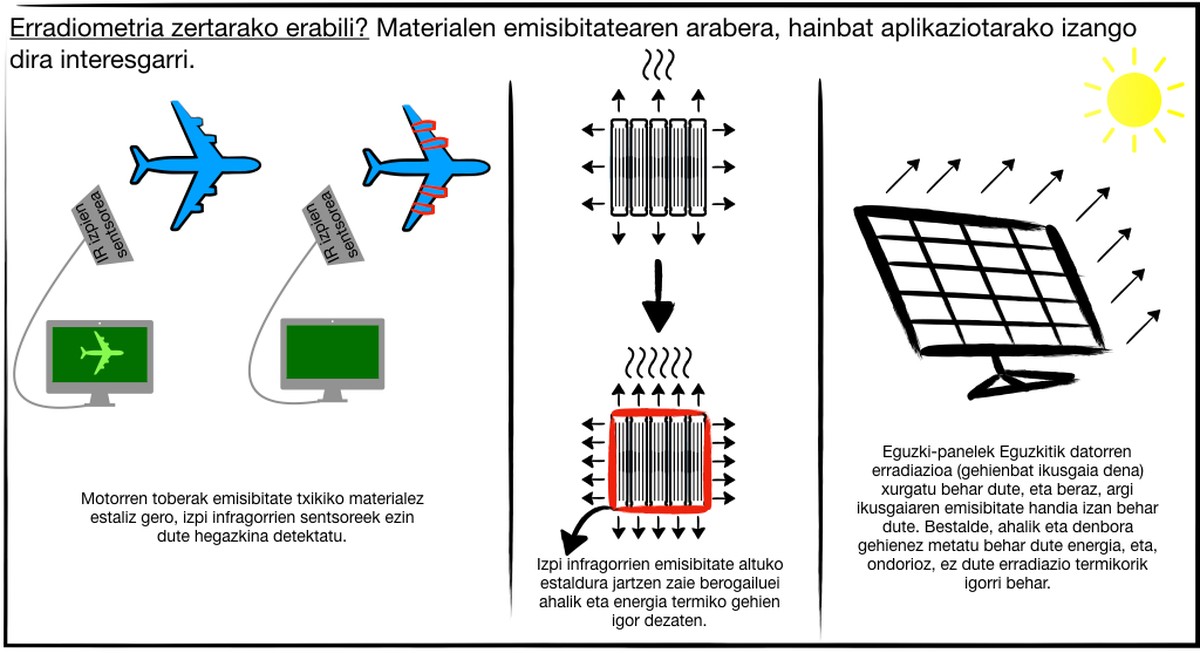
Moreover, measuring emissivity is very important for other scientific and technological applications such as reducing or increasing heat transfers, detecting bodies or not and storing energy.
Heat can be spread in three ways: by conduction, convection and radiation. Conduction is heat transfer between bodies in contact. When two bodies do not come into contact, heat transfer can occur by convection, if between both bodies there is a fluid, the conductor of heat. When there is no conductor for the propagation of heat, i.e. in vacuum, radiation is the only way to produce heat transfer. When the first two forms are not dominant, it is necessary to know well the emissivity of the body to know what heat transfers are. Examples are vacuum furnaces or materials used to insulate construction.
Bodies between ambient temperature and 2,000°C emit mostly infrared rays. Therefore, thermal sensors can be detected by infrared sensors. Therefore, if these bodies are not to be detected, they must be constructed or covered with low emissivity materials. For example, low emissivity materials are used in multi-aircraft engine hoppers to prevent infrared sensors from detecting aircraft.
Otherwise, that is, wanting to use materials of high emissivity. This is the case of the heaters, since the maximum thermal radiation is emitted at a certain temperature.
There are selective materials, that is, that emit radiation from one part of the spectrum and not radiation from another. They are very interesting for use in solar panels. Solar panels are used to obtain electrical energy through radiation from the sun. This radiation mainly refers to the visible light of the spectrum, so the panels must absorb that radiation. Therefore, they must have a great emissivity for these special wavelengths. On the other hand, the panels must store this energy as long as possible, which implies a minimum emission of thermal radiation, with a very reduced emissivity for infrared rays.
After seeing all this we can say that everything flashes, that we all blink. The truth is that the human eye cannot see the entire spectrum of radiation, so we are not aware of this phenomenon. In fact, for many technological applications it is essential to distinguish the radiation emitted by the bodies.
On the other hand, the human cortex has virtually black characteristics in the 5-20 ?m wavelength section of the emitting radiation. At that time we all (almost) shine more than anything, more than stars, more than diamonds... But all the same. In Hollywood they shine no more. There is a factor that increases emissivity, roughness. Rough materials emit more radiation than smooth ones. Therefore, as the age progresses and the skin wrinkles, instead of going off, we are going on, we are lighter.

Gai honi buruzko eduki gehiago
Elhuyarrek garatutako teknologia