Looking at the eye
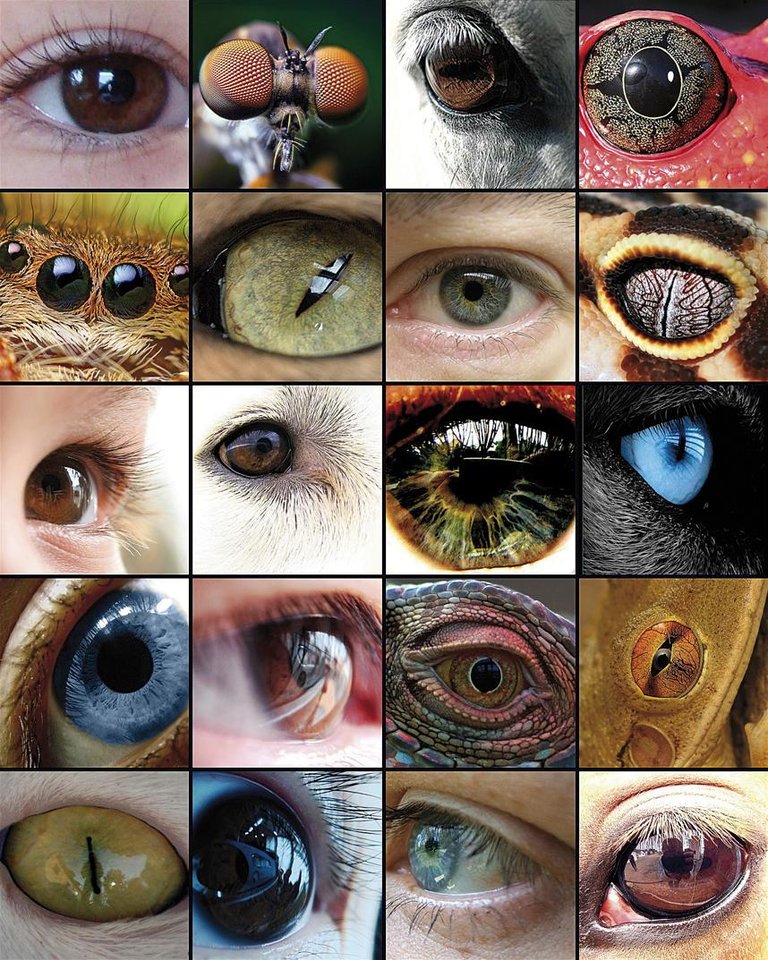
The evolution of the eye has been the basis of numerous studies, as it is a representative example of the homologous organ in the different taxa. Although some components of the eye, such as visual pigments, seem to have a common precursor, the eyes capable of producing complex images have evolved between 50 and 100 times, recurring to the same proteins and genes.
Charles Darwin himself recognized in his book The Origin of Species (1882) that he had great difficulty explaining the evolution of the eye and devoted a complete chapter to the eye under the title The Difficulties of Theory: "the fact that an organ as complex as the eye has been created by choice seems, certainly, a huge madness." But then it went on like this: "Therefore, if from a simple and imperfect eye there were the changes necessary to create the perfect and complex eye of today, as they have actually occurred, and if the animals carrying those changes were useful to the changing environment of life, the fact that our perfect complex eyes were formed by natural selection, even if it exceeds our imagination, does not seem such a revolutionary idea."
Origin of photoreceptor cells in metazoa
Photoreceptor cells are nerve cells in the retina capable of phototransduction. Photoreceptors are of great biological importance through the absorption of photons and the realization of numerous complex biochemical pathways that convert the received signal into image in the brain.
There are two hypotheses to explain the origin of the photoreceptor cells of metazoa: the first is based on differentiation and the second on symbiosis. According to the first hypothesis, the metazoa were formed from a flagellate colony, and all the cells had a photoreceptor organelle from the beginning. This photoreceptor organelles acted as an eye scar and as a response to the phototaxis that transmitted the signals through the scourge.
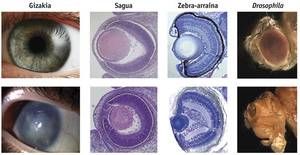
According to the symbiosis hypothesis, they have their origin in flagellates such as Volvox or Chlamydomonas, in which the photosensitive organelle is found in chloroplasts, suggesting that the perception of light is in an evolution from cyanobacteria and later integrated as chloroplast in eukaryotic cells. Another test in favor of this hypothesis, explained Greuet in 1965, describing the photoreceptor organelles of dinoflagellates such as Erythropsis and Warnovia, as developed as the human eye but added inside a single cell, with a surface similar to the cornea, a structure similar to the lenses, another similar to the retina and organelles pigments. Dinoflagellates, due to the common symbionts of corals, marine anemones and other cnidarians, could have transferred to the cnidarians the genes necessary to respond to light, which would explain the sudden appearance of the eye to the cnidarians.
According to the Sinbiontes hypothesis, sensitivity to light first arose in cyanobacteria. These cyanobacteria were internalized by red algae eukaryotic cells as primary chloroplasts. Subsequently, red algae became symbiosis with dinoflagellates as secondary chloroplasts. In some species of dinoflagellates, such as Erythropsis and Warnovia, no secondary chloroplasts have been found that are believed to have evolved and become effective photoreceptor organelles, as Greuet suggests. Finally, since dinoflagellates are common symbionts to cnidarians, they transmitted the genes of photoreceptors to them. The latter is the most uncertain step of the model, although several genes of dinoflagellates integrated in the cnidaria genome have been found.
Eye development analysis
In animals it is easy to detect mutations that affect the development of the eye and the eyeless (ey) mutation in the fly Drosophila was first discovered in 1915 by researcher Hoge. A similar mutation was found in the mouse and was called a small eye because the heterozygous animals had very small eyes, while the homozygous fetuses that died in the uterus lacked the eyes, but also the nose and some parts of the brain (Hill et al ., 1991).
In man, an inherited syndrome called aniridia produces a very similar phenotype. Small eye and aniridia genes were cloned by Walther and Gruss (1991) and Ton and their group (1991) respectively, and both responded to the extremely preserved Pax6 gene. Quiring and his team (1994) cloned Drosophila's Pax6 counterpart and were surprised that Hoge's eyeless (ey) was equal to the gene. The existence of mutations of homologous genes Small eye, aniridia and eyeless indicated that the Pax6 gene was the main control gene for ocular development in both vertebrates and invertebrates.
To demonstrate this hypothesis, the researcher WJ Gehring achieved in 1994 the mutation of the overexpression of the Pax6 gene, creating ectopic ocular structures that would express the Pax6 gene. Two of his collaborators, George Hager and Patrick Callaerts, used the Gal14 yeast transcription factor to transfer the eyeless cDNA to structures outside the eye disc.
The two collaborators managed to create ectopic visual structures on antennas, legs and wings. Subsequently, retinograms showed that some of the eyes appearing on the antennas were fully functional (Halder et al. , 1995). They also observed that the mouse Pax6 gene was able to insert it into the Drosophila to produce ectopic eyes (Gehring et al. , 1994).
These experiments showed that Pax6 was the main control gene and that this "stimulating" gene could initiate the morphogenesis of the eye, both in mammals and insects.
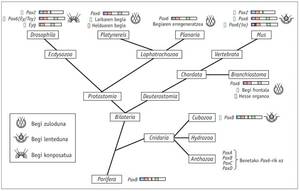
The homologous genes of Pax6 have been found in all bilateral animals studied, from the planet to man, including C. elegans. Tests have been performed with different animals and it has been proven that the Pax6 gene of all of them is capable of inducing ectopic eyes in Drosophila. The only exception to this law is the tigrine planaria Dugesia, which has gone far. As for the cnidarians, only some hydrozoans have the eyes and it is not known if the rest of the jellyfish have lost their eyes throughout evolution or have never had them.
Color Vision
Studies have shown that the color vision system is an ancestral issue, since from the beginning there was a visual pigment (called S) molded to absorb light at <500 nm and another (L) adapted to > 500 nm (Mollon, 1989). Rhodopsin, a pigment as old as these, has a maximum absorption capacity of about 500 nm and has no effect on color vision. In general, the pigments needed for color vision are found in photoreceptor cones, being functional only under the influence of light. On the other hand, rhodopsin is found in photoreceptor sticks and works in the dark.
The current vertebrates show a great variability in their visual capacity, from the density and spatial distribution of the different types of cones to the maximum absorption of the pigments of the cones (Yokoyama, 1998). At one end, most mammals contain only three pigments: two cone and rhodopsin precursors. At the other evolutionary end, chickens have six pigments.
According to studies carried out in older vertebrates, such as outlaws, the common precursor of teleosteos and amniots had four types of photopigments, as well as current birds and reptiles (Bowmaker, 1998). However, it is believed that due to the nocturnal nature of premature mammals two photopigments were lost throughout evolution, and most of the current euterium mammals only maintain photopigments S and L, that is, they are dichromatic.
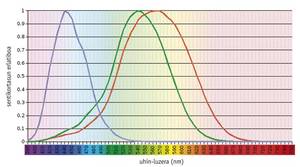
Humans and their closest primates show a model of average complexity. Humans have four visual pigments: A single member of the S family (blue), two photopigments of the L family (green or LM and red or LL) and rhodopsin.
The trichromatic vision is of great importance for primates and has been related to the advantage of finding food in forests. The most recent works have demonstrated the ecological importance of trikromacia by comparing its adaptability to the forest of bicromatic and trichromatic monkeys. In all primates, photopigment S is encoded by an autosomal gene, while photopigment L is encoded on the X chromosome. In anthropoid primates and ulary monkeys of the Old World, trichromacy arises from the duplication of the L gene, giving rise to the LM gene. However, in most New World primates, the X chromosome is the only gene in the L family. As the gene is associated with X, heterozygous females are trichromatic, and homozygous females and machas, dichromatic (green/red blinds) (Jacobs, 1998).
BIBLIOGRAPHY
Buletina
Bidali zure helbide elektronikoa eta jaso asteroko buletina zure sarrera-ontzian