Free light

Fluorescence and phosphorescence are not the same, but the difference is not great. In both cases, matter absorbs and emits light. In the case of fluorescence this occurs in a very short time, in some microseconds. In the case of phosphorescence, the time that can encompass this process is very broad: milliseconds, minutes and even hours. Therefore, in the case of phosphorescence, although there are no other sources of light, matter can continue to emit light.
Own light
Pliny Zaharra spoke about precious stones with their own light. But XVII. At the beginning of the 20th century, another singular stone aroused true scientific interest. Vincenzo Casciarolo, shoemaker and alchemist, found in the vicinity of Bologna a stone of barium sulfate that would later be known as the Bolognese stone. And there he discovered that if it was kept in the sunlight it emitted a strange light in the darkness. This aroused the imagination of the alchemists of the time. And it is that, at that time, the alchemists sought the stone of the philosophers that would serve to make gold, and of course, a magic stone of these characteristics was very encouraging.
Although it did not succeed as a philosopher's stone, curiosity about the Bolonic stone did not disappear. Galileo himself also participated in the scientific debates on this stone. For example, when Fortunius Licetus wrote that the moonlight was the same as that of the Bolonic stone, Galileo opposed it claiming that the moonlight was the reflected light of the sun.

Many research was carried out on this stone, while many researchers were discovering other substances of similar properties. All became luminous, after light or heated, etc. Therefore they were called phosphés, that is, luminous or light-holder. And today we know as phosphorescence that magical phenomenon observed by the shoemaker Casciarolo in the bolonic stone.
Despite being a phenomenon very similar to phosphorescence, fluorescence was found much later: XIX. In the 20th century. Although they first saw him in a chlorophyll solution, Sir George Gabriel Stokes (who owes much of what we know today about this subject) took the name of the fluorite phenomenon.
At the atomic level
To understand these two phenomena it is necessary to descend to the level of atoms. Basically, when a photon - or electromagnetic radiation - touches an atom, it takes the energy from the photon and gets excited. And when the atom returns to its initial relaxed state, it emits another photon, usually of less energy.
Suppose an excited electron has moved from the basic energy level S 0 to the level S 1. But within these main energy levels there are other levels. Thus, for example, the electron may rise to the third level of S 1. Immediately, this electron will tend to descend to the minimum level of S 1 and lose some energy on this path in the form of heat. Then the electron will go down to the basic level (S 0) and if on that path the energy that loses comes out as photon, we will be facing a fluorescence case.
The result of this process is that the atom that receives the radiation of a certain wavelength absorbs that first radiation and emits a radiation of greater wavelength, and therefore of less energy. This is the basis of fluorescence.
The energy levels of atoms are discrete and require a certain amount of energy to pass from one to the other. Therefore, they only absorb photons with this amount of energy. Therefore, fluorescent and phosphorescent materials emit light only with a radiation of a certain wavelength, and in turn, the light they emit will be of a certain wavelength. Or, more specifically, a specific range of wavelengths. In fact, within the basic energy level electrons do not always fall to the same level. And therefore, not all emitted radiation will be of the same wavelength. The wavelength in which absorption and emission occurs depends on the components and the state of the material.

Since the emitted radiation is less than the absorbed radiation so that the emitted one has a visible spectrum, normally the atoms must aspire to ultraviolet rays. Therefore, fluorescence usually appears in most cases under ultraviolet radiation. However, the absorbed and emitted can also belong to the same spectrum, and sometimes it also costs to separate them.
Forbidden route
In the case of phosphorescence the process is very similar. But the excited electrons are 'trapped' in another special situation. In fact, in this case, it is said that the passage of this peculiar state to a basic energy level is quantically prohibited. This does not mean that it does not pass, but the probability is much lower. Therefore, the process is lengthened and, after eliminating the source of radiation, the phosphorescent material continues to emit light.
However, most phosphoresents are relatively fast emitters that emit light in a few milliseconds. In the case of others, however, the process can be lengthened: minutes and even hours, so they are usually materials that give light in the dark. This is the case of Bolognese stone. And that is also what happens on the hands and numbers of many clocks, or to stick on the ceiling on those stars and planets, etc.
But phosphorescence has another widely used application. What we see in conventional televisions or on the screens of desktop computers is precisely phosphorescence, if these screens work by means of a cathodic ray tube. In these screens three phosphorescent components are used to create colored images: zinc sulfide is combined with copper and aluminum, obtaining a green component; for blue, zinc sulfide is mixed with a little silver; and finally, the red color is obtained by activating the sulfide of yttrium oxide with the European.

At home, disco and laboratory
Fluorescence is currently fully domesticated. In fact, it is very common to use fluorescent tubes for lighting any type of building. These tubes contain mercury inside and the tube walls a fluorescent coating inside. When an electrical discharge occurs between the electrodes of the tube, the electrons excite the mercury atoms and these emit ultraviolet radiation.
That radiation is not visible, and if only that happens, we would not see the light. But this ultraviolet radiation emitted by the excited mercury atoms in turn excites the atoms of the fluorescent tube lining, which emit the visible light coming out of the fluorescent tube.
And the chain of atoms that are excited can be longer. In fact, other fluorescent lamps are used simultaneously to produce fluorescence. They are similar to conventional fluorescent tubes, but with different coverage, and emit some visible light and, above all, a nearby ultraviolet radiation, called “black light”.
This near ultraviolet radiation is usually of a wavelength greater than 350 nm, very close to the visible spectrum, so it has no adverse effects on ultraviolet rays of lower wavelength. These lamps are used to highlight fluorescent materials in the dark. For example, they are common in discos to induce the fluorescence of polyester that is usually present in white tissues.
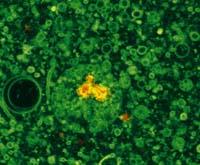
In addition to at home and disco, fluorescence is also important in laboratories. Fluorescence has many applications in science. It is widely used in biochemistry and medicine to detect molecules, cells or tissues. Some molecules may have their own fluorescence, but in many cases fluorescent marks --fluorofores- are used to mark what is intended to be detected.
For example, by adding a fluoroforum to antibodies, the antigen of this antibody can be found in a sample. Fluorescence microscopes are used for this purpose. These microscopes illuminate the sample with ultraviolet rays that allow visual observation of fluorescence or by a monitor.

Fluorescence has more scientific applications every year. But we have seen that in everyday life fluorescence and phosphorescence are more common than we might think at first. If you are using one or both to read, I don't know, but to write this article both were essential.
Buletina
Bidali zure helbide elektronikoa eta jaso asteroko buletina zure sarrera-ontzian











