Related information
1993/02/01 Azkune Mendia, Iñaki - Elhuyar Fundazioa Iturria: Elhuyar aldizkaria
Italian physicist born in Turin, Piedmont, 9 June 1776.
He studied law and in 1800 began studying mathematics and physics. As he worked in depth the sciences, teaching. In 1809 he was appointed professor of mathematics and physics at the royal college of Vercelli and from 1820 professor of higher physics at the University of Turin.
According to Gay-Lussac's finding, as the temperature increased, all gases were distributed in the same proportion, and Avogadro, studying it, had an idea. It considers that all gases should have the same number of particles per unit of volume at a certain temperature. This idea was transferred in 1811 and he commented very carefully that the particles mentioned did not have what the individual atoms consisted of. There could also be atomic combinations (which we call molecules today).
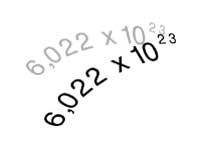
From there he easily explained the law of combining volumes of Gay-Lussac. XIX. At the beginning of the century Ritter electrolyzed water collecting oxygen and hydrogen separated. He was able to verify that the oxygen volume was half that of hydrogen. Avogadro then demonstrated that, applying its hypothesis, the water molecule had two hydrogen atoms and one oxygen atom. If the total weight of the collected oxygen was eight times greater than that of hydrogen, the oxygen atom was sixteen times heavier (and not, according to Dalton, eight times heavier).
However, Avogadro's ideas were not taken into consideration in the coming decades. Dalton abandoned them and the most famous chemist of the time, Berzelius, came to cancer. As a result, the chemicals had large mixtures, inseparable atoms, molecules, atomic weights and molecular weights.
Estanislao Cannizzaro was the first to consider Avogadro, two years after his death, and it was then that he achieved the reputation that tourist science denied him in his life.
It is now known as Avogadro, because the number bearing its name serves to calculate how many atoms (or molecules) the atomic weight expressed in grams has. Carbon oxide (IV), for example, has a molecular weight 44 and in 44 grams of this gas there will be as many molecules as the number of Avogadro, that is, 6,022x1023 molecules (602,600 trilion molecules).
It is also used in chemistry to define mole. A mole of substance is the amount containing 6,022x1023 units of that substance.
Avogadro Quaregna and Count of Ceretto died in Turin on 9 July 1856.

Gai honi buruzko eduki gehiago
Elhuyarrek garatutako teknologia





