At the limit of absolute zero

XVIII. At the beginning of the 20th century, physicist Guillaume Amontons concluded that, while studying the relationship between temperature and gas pressure, a sufficient decrease in temperature would mean the disappearance of pressure, so the temperature should have a minimum. Thus the concept of absolute zero appears for the first time. And, according to Amontononons' estimates, this limit was at -240°C.
In the following century, Lord Kelvin performed more precise calculations and established an absolute temperature scale. The degrees of this scale have the same value as those of the Celsius scale, but the Kelvin scale starts from the absolute zero. The absolute zero is therefore 0 K, and on the Celsius scale -273,15ºC.
To understand the absolute zero, the first thing to know is temperature. Temperature is the kinetic energy of the atoms of matter. When matter is hot, atoms or molecules move quickly, that is, they have a great kinetic energy.
When matter is solid, atoms are bound together and motion is limited. In a gas, however, we can imagine atoms as pelotites flying in all directions. The hotter, the faster the balls move. Thus, the temperature indicates the average speed of these balls.
The decrease in temperature is therefore a buffer of atoms, and if it does enough, a point would come where atoms would remain completely. Since there can be no speeds below zero, there can be no lower temperature. There is the absolute zero.
But it is also impossible to completely stop an atom, at least according to the uncertainty principle of Heisenberg. Therefore, absolute zero is an unattainable limit. The third law of thermodynamics also says that it is impossible to reach absolute zero in a finite number of steps. However, scientists have tried to get as close as possible and have also done enough.
Approaching zero

XIX. In the 19th century, researchers discovered that the liquefaction of several gases, such as hydrogen, oxygen and helium, allowed to reach very low temperatures. And by 1908 a temperature of 4.2 K had been reached.
At these cold temperatures, some materials get 'superpowers'. Some metals, for example, become superconductors, i.e. their resistance to electric current decreases to zero. There are also superfluids, liquids without viscosity. This is the case of liquefaction of helium.
But perhaps the most curious phenomenon is that of the condensed Bose-Einstein. It is a new state of matter in which all atoms are at a quantum level of minimal energy.
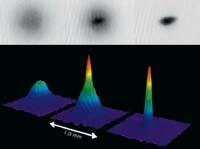
Although theoretically known earlier, the first condensed Bose-Einstein was obtained by Eric Cornell and Carl Weiman in 1995. For this it was necessary to reach temperatures much lower than those until then. The rubidium atoms first cooled by laser and then continued to cool down by "evaporation" to 170 nanocelvin (10-9 K). Months later, Wolfgang Ketterle also got another Bosé-Einstein condensate with sodium atoms. These three physicists were awarded the 2001 Nobel Prize in Physics for their work around the condensed Bose-Einstein.
A team of researchers from the Massachusetts Institute of Technology, led by Ketterle, achieved in 2003 the lowest temperature record using the same technique. The cesium atoms managed to cool to 450 chips, that is to say, to 0.0000000000045 on the absolute zero.
Laser cooling
To approach both the absolute zero, as has been said, the atoms are cooled first by laser. The laser is a photon beam in which all the photons of a laser are equal, that is, they have the same energy.
When a photon of a certain color or wavelength touches an atom, it absorbs the photon and then emits it. The photon has a moment and when aspiring that moment affects the atom. That is, by sucking a photon an atom that goes straight to the laser, the moment of the atom decreases as much as the moment of the photon. Or what is the same, the photon pushes the atom in the opposite direction, so it cushions it. This impulse is very small. A photon can cushion 3 cm/s a sodium atom and the sodium atoms have an average speed of 570 m/s at room temperature. Paralyzing atoms with photons means stopping a bowling ball by throwing ping-pong balls. But with a laser, the atom can absorb 10 million photons per second.
On the other hand, when emitting the photon, the atom again suffers a push, but in this case the photon can come out in any direction, at random. Thus, the moment or push due to the emission after several absorption processes, will be 0. Therefore, the absorption of photons by atoms in the opposite direction to their movement can cause their absorption. On the contrary, if a photon attracts a moving atom from behind, the moment of the atom would increase, that is, it would accelerate and warm up.
And since atoms move in all directions in a gas, how can the photon be absorbed in the opposite direction to the movement of atoms? There comes into play the Doppler effect. According to this effect, an atom moving towards the laser would see the color shifted to the blue, while one moving away would see more reddish than it is. This change of color or wavelength also depends on the speed.
In this way, the laser of a certain color will only affect the fastest atoms you face, not the slower or not in the right direction. As atoms are cushioned, it will be necessary to reduce the wavelength of the laser to further attenuate atoms. And if we place lasers everywhere, this effect is achieved in all directions.
Traps for atoms
Another problem is that these atoms, although attenuated, are still moving and if they touch the walls they will reheat. Laser traps are used to prevent this. In laser traps, lasers always push atoms toward the center, and the atom that is going to leave the center always finds another laser that pushes it back toward the center. A magnetic field is formed to change from center to exterior. The magnetic field slightly changes the color of the laser and what previously happened with the Doppler effect happens again. But if before the push caused by the laser depended on the speed and direction of the atom, it will now depend on the position.
Thus, atoms can cool to a point, about 0.0001 K. But if you want to cool more, you have to leave the photons aside. In fact, photons continue to give little push to the dampened atoms, movements that do not allow to reach the desired temperature.

To keep reducing the temperature, first you have to get the atoms to stay in the center without touching the hot walls, but without the help of the photons. This is done with another trap, a magnetic trap. A powerful magnetic field is used that acts directly on atoms, and if the magnetic field is appropriate, atoms can be kept in the center.
Once obtained, you can continue with the "evaporation" by cooling these cold atoms. In this case, the principle is the same as when the broth is cooled. The most energetic particles in the broth are vaporized. In doing so they carry some heat and the atoms remaining in the balance cool. For with cold atoms the same thing is done; those who have more energy are allowed to escape the magnetic trap. To do this, the magnetic field decreases progressively, those with more energy are removed and those that remain cool more.
Thus the condensate Bose-Einstein is obtained. It is not easy to understand what happens in this new type of matter, since it has nothing to do with common matter. All atoms are in the same quantum state at the lowest level. This means that all atoms are exactly the same and move all at once, in perfect synchronization. Therefore, it is impossible to separate one atom from the other. In addition, all atoms occupy the same place, all form a common mass. That is why there are those who have called them superatoms.
The coldest matter ever obtained is the condensed Bosé-Einstein cesium or cesium superatom. However, this superatom still has movement, has energy and therefore has temperature. It is not at all cold, but it is there, at the limit of absolute zero.


Buletina
Bidali zure helbide elektronikoa eta jaso asteroko buletina zure sarrera-ontzian