Molecules are also waves

Problems in defining the basic components of matter, as it is very difficult to say exactly what they are. How is a molecule? And an atom? And an electron? If we were small, could we catch a proton in our hand? Would we keep a neutron?
Physicists talked a lot about this problem of character about 65 years ago. Then the nature of the electron was questioned, a particle that acted very differently according to the type of experiment.
For example, the electron had the behavior of particles in the case of the photoelectric effect, in which an electron and a photon collided with each other, bouncing both as if they were balls. For this experiment, Einstein received the Nobel Prize in Physics in 1905.
However, by passing a beam of electrons through a pair of slots they diffuse, that is, as if they were water waves, the beams produce new waves at the exit of the slits.
According to the first experiment, electrons are particles and, according to the second, waves. Finally, the French physicist Louis de Broglie concluded that electrons are both particles and waves; so to speak, one character did not discard the other. For this work he was awarded the Nobel Prize in 1929.
Now the molecules
De Broglie's theory applies not only to the electron, but also to the photon, proton, neutron and all particles of this size. What's more, it also works with the entire atom, that is, sometimes it collides in the way of a ball and other times it diffracts.
This theory has been generalized by physicists. A wave corresponds to all solid bodies. Whether it is an atom, a stone or a planet, it is the same; a wave corresponds to all of them. But the bigger it is, the more body character predominates. We would not say that a planet is the wave, but the body because, studying it in any way, it acts as a body. But from what size is the effect of being a wave "lost"?
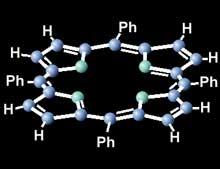
In recent times there has been an experiment of slits with bundles of molecules that have found the same behavior as the atom or the smaller particles. Now larger and larger molecules are being tested in this experiment, seeking size limitation.
The last one they have tested has been tetrafenil porphyrin, a large molecule in hemoglobin and chlorophyll. A set of prophyrin is also broken by passing through both slits, so it behaves in waveform.
For physicists, for the moment, the limit is around, in molecules of two nanometers. They will also test the larger ones, which can alter the well-known border. However, handling larger molecules presents difficulties. We will see how far they arrive next time.
Buletina
Bidali zure helbide elektronikoa eta jaso asteroko buletina zure sarrera-ontzian