Glass: neither glass nor liquid

It has been proven that the glass components form 20-sided polyhedra (ikosaedros) when molten glass is cooled, and that the appearance of these polyhedra makes the glass structure the one it contains.
People who have been working on matter physics for some time have been very curious about the structure of glass. In fact, they saw that glass does not correspond to any of the usual conditions of other materials, since glass is not solid, liquid, or gas. In short, we can say that glass is in an intermediate state between solid and liquid.
Well, yes, the glass is solid, but it doesn't have the characteristics that solids usually have. Solid-state substances usually form crystals, that is, the components occupy the space in an orderly and symmetrical way. In a liquid state, the components of a certain substance have no order, as they are not related to each other.
The glass has a solid shape, so its components do not move but do not acquire glass shape. Somehow, glass components are like cars that would be in constant congestion, trapped in chaos.
A lot of work to discover
The English physicist Charles Frank claimed for the first time 50 years ago that the glass components form icosahedra. In his opinion, these polyhedra find it impossible to crystallize correctly, since by their appearance they cannot fill the space symmetrically and orderly.
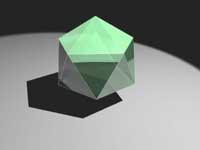
Said yes, but he could not prove at all what was said. Now the researchers have been able to confirm what Frank announced. However, they have not seen closely the solidification of glass.
They knew it is impossible to observe it with the tools they have now available. Microscopes (optical microscopes) that allow the three-dimensional observation of the process are unable to visualize the atoms separately, since they are too small, while in microscopes that allow the visualization of atoms it is impossible to observe three-dimensional images.
Therefore, they followed another path. When the molten glass was cooled, groups of molecules were used that suffer the same process as the glass components: the colloids. Colloids are very small particles (small but also visible under an optical microscope) found in a fluid. For example, mayonnaise and gelatin are made from colloids.

Colloids in a liquid state cooled and observed what was happening. They observed that the Ikosahedrons were forming, and that the more they cooled the more icosahedrons there were, and that the Ikosahedrons formed more and more chaos.
Scientists claim that this discovery has opened a way for them to create new materials. For example, they believe that if they follow this procedure with metals they can create “metal beirakarak’. These metals would be much more flexible than metals with crystalline structure and could be used for various uses, such as aircraft wings.
Current metals are more fragile because they are made up of glass granules attached to each other and, of course, because separating the edges between grains is easier than breaking crystals. Glass metals, for their part, would not have this edge.
Published in 7K.
Buletina
Bidali zure helbide elektronikoa eta jaso asteroko buletina zure sarrera-ontzian








